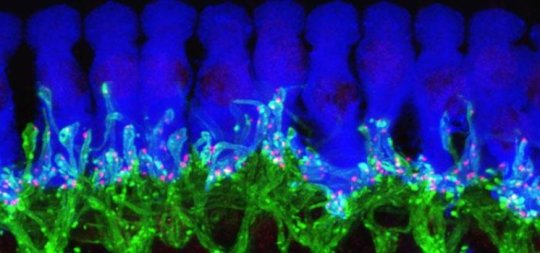
This research shows that it is possible to design gene therapies for the ear that are carefully targeted at supporting cells, an essential first step in applying targeted gene therapies to treat hearing loss in humans.
Recent Research From ERG Alumni
The effect an Emerging Research Grant has on the hearing and balance fields is evident by the discoveries our funded researchers continue to make. Here are recent highlights by ERG alumni, each getting a boost at the start of their career with a grant from HHF.
Molecular Barriers to Overcome for Hair Cell Regeneration in the Adult Mouse Cochlea
The research suggests that reprogramming with multiple transcription factors is better able to access the hair cell differentiation gene regulatory network, but that additional interventions may be necessary to produce mature and fully functional hair cells.
John Brigande provides commentary: Hearing in the mouse of Usher
Oregon Health & Science University
The March issue of Nature Biotechnology brings together a set of articles that provide an overview of promising RNA-based therapies and the challenges of clinical validation and commercialization. In his News and Views essay, “Hearing in the mouse of Usher,” John V. Brigande, Ph.D., provides commentary on two studies in the issue that report important progress in research on gene therapy for the inner ear.
One in eight people in the United States aged 12 years or older has hearing loss in both ears. That figure suggests that, if you don’t have hearing loss, you likely know someone who does. Worldwide, hearing loss profoundly interferes with life tasks like learning and interpersonal communication for an estimated 32 million children and 328 million adults worldwide. Inherited genetic mutations cause about 50 percent of these cases.
The challenge in developing gene therapy for the inner ear isn’t a lack of known genes associated with hearing loss, but a lack of vectors to deliver DNA into cells. Brigande, associate professor of otolaryngology and cell, developmental, and cancer biology at the OHSU School of Medicine, provides perspective on companion studies that demonstrate adeno-associated viral vectors as a potent gene transfer agent for cochlear cell targets.
The first study demonstrates safe and efficient gene transfer to hair cells of the mouse inner ear using a synthetic adeno-associated viral vector that promises to be a powerful starting point for developing appropriate vectors for use in the human inner ear. The second study demonstrates that a single neonatal treatment with this viral vector successfully delivers a healthy gene to the inner ear to achieve unprecedented recovery of hearing and balance in a mouse model of a disease called Usher syndrome. Individuals with Usher syndrome type 1c are born deaf and with profound balance issues and experience vision loss by early adolescence. The research teams were led by scientists from the Harvard School of Medicine.
Brigande sees these new studies as potentially spurring investment and kickstarting the development of new approaches to correct a diverse set of deafness genes.
Hearing Restoration Project consortium member John V. Brigande, Ph.D., is a developmental neurobiologist at the Oregon Hearing Research Center. He also teaches in the Neuroscience Graduate Program and in the Program in Molecular and Cellular Biology at the Oregon Health & Science University. This blog was reposted with the permission of Oregon Health & Science University.
Hearing Beyond the Hair Cell
By Yehoash Raphael, Ph.D.
Recently, it became clear that loud signals can also damage the connecting interface between the hair cell and the auditory nerve. This interface is the synapse. When the synapse is disrupted, hearing is impaired even without the loss of hair cells, leading to a condition called synaptopathy.
Experiments using transgenic mice showed that elevating levels of a specific molecule called NT3 in the area of the synapse can heal synaptopathy caused by exposure to loud noise. Since transgenic technology is a research tool not applicable for clinical use on humans, it is now necessary to design methods for elevating NT3 in human ears, leading to repair of synaptopathy. This is an important task, because if left untreated, synaptopathy progresses to include nerve cell death and permanent hearing deficits.
One potential way to increase NT3 concentration in the cochlea is by the use of gene transfer technology, which is based on infecting cochlear cells with viruses that are engineered to secrete NT3 and not cause infections. A potential risk of this method is that the site of NT3 is not restricted to the area of the synapses affected by the synaptopathy; NT3 can influence other types of cells.
In my lab at the University of Michigan, we tested the outcome of injecting such viruses on the structure and function of normal (intact) ears. We determined that the procedure resulted in the deterioration of hearing thresholds, and the auditory nerve and its connectivity to the hair cells were also negatively affected.
This negative outcome indicates that treatment of synaptopathy should be based on a more specific way to provide NT3 in an area restricted to the synaptic region. My work with the Hearing Restoration Project is dedicated to optimization of gene transfer technology in the cochlea, and may assist in finding more detailed methods for NT3 gene transfer that better target affected cells.
More information on Dr. Raphael’s research can be found in his report, “Viral-mediated Ntf3 overexpression disrupts innervation and hearing in nondeafened guinea pig cochleae,” published in the journal Molecular Therapy—Methods & Clinical Development on August 3, 2016.
Yehoash Raphael, Ph.D., is the The R. Jamison and Betty Williams Professor at the Kresge Hearing Research Institute, in the Department of Otolaryngology–Head and Neck Surgery at the University of Michigan.
Scientists restore hearing in noise-deafened mice
By the University of Michigan Health System
Scientists have restored the hearing of mice partly deafened by noise, using advanced tools to boost the production of a key protein in their ears.
This microscope image of tissue from deep inside a normal mouse ear shows how ribbon synapses (red) form the connections between the hair cells of the inner ear (blue) and the tips of nerve cells (green) that connect to the brain.
Credit: Corfas lab - University of Michigan
By demonstrating the importance of the protein, called NT3, in maintaining communication between the ears and brain, these new findings pave the way for research in humans that could improve treatment of hearing loss caused by noise exposure and normal aging.
In a new paper in the online journal eLife, the team from the University of Michigan Medical School's Kresge Hearing Research Institute and Harvard University report the results of their work to understand NT3's role in the inner ear, and the impact of increased NT3 production on hearing after a noise exposure.
Their work also illustrates the key role of cells that have traditionally been seen as the "supporting actors" of the ear-brain connection. Called supporting cells, they form a physical base for the hearing system's "stars": the hair cells in the ear that interact directly with the nerves that carry sound signals to the brain. This new research identifies the critical role of these supporting cells along with the NT3 molecules that they produce.
NT3 is crucial to the body's ability to form and maintain connections between hair cells and nerve cells, the researchers demonstrate. This special type of connection, called a ribbon synapse, allows extra-rapid communication of signals that travel back and forth across tiny gaps between the two types of cells.
"It has become apparent that hearing loss due to damaged ribbon synapses is a very common and challenging problem, whether it's due to noise or normal aging," says Gabriel Corfas, Ph.D., who led the team and directs the U-M institute. "We began this work 15 years ago to answer very basic questions about the inner ear, and now we have been able to restore hearing after partial deafening with noise, a common problem for people. It's very exciting."
Using a special genetic technique, the researchers made it possible for some mice to produce additional NT3 in cells of specific areas of the inner ear after they were exposed to noise loud enough to reduce hearing. Mice with extra NT3 regained their ability to hear much better than the control mice.
Now, says Corfas, his team will explore the role of NT3 in human ears, and seek drugs that might boost NT3 action or production. While the use of such drugs in humans could be several years away, the new discovery gives them a specific target to pursue.
Corfas, a professor and associate chair in the U-M Department of Otolaryngology, worked on the research with first author Guoqiang Wan, Ph.D., Maria E. Gómez-Casati, Ph.D., and others in his former institution, Harvard. Some of the authors now work with Corfas in his new U-M lab. They set out to find out how ribbon synapses -- which are found only in the ear and eye -- form, and what molecules are important to their formation and maintenance.
Anyone who has experienced problems making out the voice of the person next to them in a crowded room has felt the effects of reduced ribbon synapses. So has anyone who has experienced temporary reduction in hearing after going to a loud concert. The damage caused by noise -- over a lifetime or just one evening -- reduces the ability of hair cells to talk to the brain via ribbon synapse connections with nerve cells.
Targeted genetics made discovery possible
After determining that inner ear supporting cells supply NT3, the team turned to a technique called conditional gene recombination to see what would happen if they boosted NT3 production by the supporting cells. The approach allows scientists to activate genes in specific cells, by giving a dose of a drug that triggers the cell to "read" extra copies of a gene that had been inserted into them. For this research, the scientists activated the extra NT3 genes only into the inner ear's supporting cells.
The genes didn't turn on until the scientists wanted them to -- either before or after they exposed the mice to loud noises. The scientists turned on the NT3 genes by giving a dose of the drug tamoxifen, which triggered the supporting cells to make more of the protein. Before and after this step, they tested the mice's hearing using an approach called auditory brainstem response or ABR -- the same test used on humans.
The result: the mice with extra NT3 regained their hearing over a period of two weeks, and were able to hear much better than mice without the extra NT3 production. The scientists also did the same with another nerve cell growth factor, or neurotrophin, called BDNF, but did not see the same effect on hearing.
Next steps
Now that NT3's role in making and maintaining ribbon synapses has become clear, Corfas says the next challenge is to study it in human ears, and to look for drugs that can work like NT3 does. Corfas has some drug candidates in mind, and hopes to partner with industry to look for others.
Boosting NT3 production through gene therapy in humans could also be an option, he says, but a drug-based approach would be simpler and could be administered as long as it takes to restore hearing.
Corfas notes that the mice in the study were not completely deafened, so it's not yet known if boosting NT3 activity could restore hearing that has been entirely lost. He also notes that the research may have implications for other diseases in which nerve cell connections are lost -- called neurodegenerative diseases. "This brings supporting cells into the spotlight, and starts to show how much they contribute to plasticity, development and maintenance of neural connections," he says.
In addition to Corfas, Wan and Gómez-Casati, who now works in Argentina, the research was performed by Angelica R. Gigliello, and M. Charles Liberman, Ph.D. director of the Eaton-Peabody Laboratories of the Massachusetts Eye and Ear Infirmary. The research was supported by the National Institute on Deafness and Other Communication Disorders (DC004820, DC005209) and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD18655), both part of the National Institutes of Health, and by the Hearing Health Foundation.
The above post is reprinted from materials provided by University of Michigan Health System.
We need your help in funding the exciting work of hearing and balance scientists.
To donate today to Hearing Health Foundation and support groundbreaking research, visit hhf.org/name-a-grant.
Ask the Scientist: Gene Therapies and Hearing
By Peter G. Barr-Gillespie, Ph.D.
A DNA double helix
National Human Genome Research Institute
Recently, Hearing Health Foundation (HHF) has received several questions regarding the Reuters report on gene therapies for hearing. There are two separate but related topics raised in this article. As the scientific research director of HHF’s Hearing Restoration Project, which since 2011 has been uncovering concrete discoveries toward a biologic cure for hearing loss and tinnitus, I want talk about each individually, and then discuss what I interpret they mean together.
The article first presents the Science Translational Medicine paper from Jeffrey Holt’s lab. This is very much a proof-of-principle report, focused on an animal model and using a time for delivery of the corrected gene that is extremely early in development (equivalent to a 5-to-6-month-old human fetus). It is important to point out that their strategy will only correct one type of genetic hearing loss and genetic hearing loss from mutations in other genes will require related but different strategies. Nevertheless, this is an exciting example of modeling gene therapy in animals, and represents a logical progression toward that goal in humans.
The article then moves on to reference the Novartis trial. For this trial, they are using a similar technical strategy, viral delivery of a gene, but they are targeting people—those who have lost their hearing through non-genetic means, such as noise damage, aging, or infections. The gene they are delivering, known as ATOH1, may stimulate production of new hair cells; it is a gene that is essential for formation of hair cells during development, and in some experimental animal models, delivery of the gene can lead to production of a few hair cells in adult ears.
That said, many people who I have talked to in the field who work with experimental models of hair cell formation using ATOH1, including members of our Hearing Restoration Project consortium, believe that this trial is premature. By and large, the animal models do not support the trial; most suggest that there will be few hair cells formed and little hearing restored. While we can hope for a little bit of hearing recovery, we are concerned about toxic responses to the gene delivery using viruses. Personally, while I think it would be truly fantastic if the Novartis trial works, at this moment in time I don’t think the rewards yet outweigh the considerable risks being imposed on a human (include safety during the procedure and potential side effects afterward).
Still, the Novartis trial will tell us about the safety of viral delivery into the ears of humans, and knowing that is critically important. I think the most likely outcome is that we will learn whether the strategy the Novartis trial used to deliver the gene is safe. Unfortunately, if we don’t see improved hearing, we won’t know why—did the gene not get to the right place, or does it just not work?
Technical aspects of gene delivery are what ties together the Novartis work and the Holt lab work. Both use viruses for delivering genes, and together the results from these and others will let us know, from a procedural standpoint, how we can deliver genes to the ear. I think it is unlikely that delivering just ATOH1 will do the trick of restoring hearing; it may be that we need to deliver other genes or to use drugs to overcome the block we see to making new hair cells.
So while these are exciting reports to hear about, especially that Novartis is actually carrying out a trial in humans, it is still premature to think that this is going to be a viable strategy for restoring hearing. This is why Hearing Health Foundation's Hearing Restoration Project is doing everything possible to accelerate the pace of its research.
Hair cell regeneration is a plausible goal for the treatment of hearing and balance disorders. The question is not if we will regenerate hair cells in humans, but when. Your financial support will help to ensure we can continue this vital research and find a cure in our lifetime! Please help us accelerate the pace of hearing and balance research and donate today. Your HELP is OUR hope!
If you have any questions about this research or our progress toward a cure for hearing loss and tinnitus, please contact Hearing Health Foundation at info@hhf.org.
The Path to a Cure for Hearing Loss and Tinnitus
By Laura Friedman
On May 21, 2015, Hearing Health Foundation hosted its first live-video research briefing as part of our effort to provide regular updates on our research programs and progress. Through these briefings, our goal is for our attendees to obtain new information and understanding about hearing loss, prevention and research toward a cure.
During this inaugural research briefing, Dr. Peter Barr-Gillespie, Scientific Director, Hearing Restoration Project presented the Hearing Restoration Project (HRP). The HRP was founded in 2011 and is the first and only international research consortium focused on investigating hair cell regeneration as a cure for hearing loss and tinnitus. The overarching principle of the consortium is collaboration: open sharing of data and ideas. The HRP consortium consists of 14 of the top investigators in the audiological space, as well as a scientific director, Dr. Barr-Gillespie.
We wanted to share with you highlights from the presentation, which is available to watch with live captioning or to read with notes summarizing each slide.
History of Hearing Health Foundation
Founded in 1958, established reputation for pioneering breakthroughs in hearing and balance research.
Early supporters of the revolutionary cochlear implant. Today, over 220,000 children and adults benefit.
Advocated for the passage of Universal Newborn Hearing Screening legislation in the 1990s. Today, 97% of newborns are tested for hearing loss at birth.
The Emerging Research Grants Program provides seed funding for researchers in hearing and balance science such as discoveries in hair cell regeneration, tinnitus, hyperacusis, and Ménière’s research.
The Challenge
In the past century, the primary treatment for hearing loss has been hearing aids and cochlear implants. While these have been very successful treatments, they have limitations.
For this century, we have a number of different avenues for more effective therapy.
Preventing the damage to the hair cells to preserve hearing. By generating greater awareness of the effects of hearing loss, we aim encourage people of all ages to protect their ears.
Gene therapy, targeting those who have lost hearing due to genetic disorders.
The majority of people who have lost hearing have done so through noise damage or aging, and may be candidates for hair cell regeneration/restoration.
HRP Consortium History & Model
One of the key facets of the HRP’s approach is that we use three different animal models for studying hair cell regeneration
Two of those models, the chick and the zebrafish, show robust hair cell regeneration.
f you damage the hair cells of a chick or a fish, within a short time—only a day or two for the fish, a few weeks for the chick—the hair cells come back; new hair cells are formed.
So, that's spectacular, because it tells us that animals are capable of regenerating hair cells.
y contrast, the mouse is our other experimental model. Like in the human, the mouse shows no hair cell regeneration after a few days following birth.
You can damage the hair cells in the mouse and as far as we can tell, nothing much happens in terms of restoring hair cells. So, if we can figure out how to regenerate hair cells in the mouse, then we will be able to regenerate hair cells in people.
HRP Strategic Research Plan
Our strategic plan consists of three separate phases. We have already made a lot of progress on Phase 1 and we have initiated Phase 2:
Phase 1 – Discovery research: Compare the fish, chick, and mouse to discover pro- or anti-regeneration pathways and determine supporting cell fates.
Phase 2 – Pathway validation: Verify pathways using fish, chick, and mouse models and describe regeneration strategies.
Phase 3 – Develop therapies and treatment options: Identify drugs that trigger hair cell regeneration in the mouse model.
Progress To-Date
Progress on Phase 1: We've identified a variety of candidates for hair cell regeneration and the pathways that are necessary.
We have too many, so we really are continuing to use bioinformatics methods to winnow down and determine which are most important.
We have definitively shown, at least in the mouse, the specialized supporting cells remain.
We know now what our target cells are for triggering hair cell regeneration.
Phase 2 has begun, but we haven’t stopped Phase 1:
We've got multiple approaches to try and see whether or not we can block regeneration in the fish and chick or stimulate regeneration in the mouse.
Phase 3 is in sight:
Experimental models from Phase 2 will be used to screen for drugs—using the mouse first
The Next Five Years
With your help, we can continue to quicken the pace towards a cure. Here’s our plan for the next five years:
Phase 1 will continue: more candidate generation for Phase 2
Phase 2 (pathway verification) already initiated in zebrafish, mouse, chick (low throughput)
Phase 2 must be scaled up: many more genes, combinatorial approaches; cell lines for screening
Phase 3 (drug screening) requires the right screening model, which will come out of Phase 2.
The Future is Very Bright – But we need your support!
Hair cell regeneration is a plausible goal for eventual treatment of hearing and balance disorders. The question is not if we will regenerate hair cells in humans, but when. However, we need your support to continue this vital research and find a cure! Please make your gift today.
The Case for Genetic Testing
By Yishane Lee
Genetic causes account for roughly half of hearing loss cases in infants, according to the Centers for Disease Control and Prevention. Many cases of progressive hearing loss that occur after infancy and childhood also have a genetic component.
At least 1,000 mutations in 64 genes linked to hearing loss have been identified. Thanks to rapid advances in genetic sequencing, identifying virtually all the genetic causes of hearing loss may occur within the decade, according to a recent report in the journal Genetic Testing and Molecular Biomarkers.
Researchers are using “targeted resequencing” to locate gene mutations in certain regions in the human genome that are linked to diseases much more quickly than sequencing the entire genome. In our Fall 2013 issue of Hearing Health magazine, Xue Zhong Liu, M.D., Ph.D., reviews the advances in sequencing technology and how this will affect the future treatment of hearing loss.
Because genetics can play such a significant role in hearing loss, genetic testing can answer questions you have about the cause of your or a loved one’s hearing loss. If the testing uncovers a mutation, it can help explain the hearing loss, its severity or progression, and whether other symptoms may become apparent. For instance, a person with Usher syndrome has not only hearing loss but also eventual blindness. You can proactively take steps to manage treatment and outcome. Knowing the genetic cause of a hearing loss can also help you predict whether the condition will be passed along to your children, or whether the children of other family members may have the condition.
Last summer, Hearing Health magazine presented an overview of genetic causes of hearing loss, including Connexin 26 disorder. This is the most common cause of congenital hearing loss not related to a syndrome (with other symptoms, such as a thyroid problem). Mutations in the GJB2 gene affect development of the cochlea in the inner ear. Everyone carries two copies of the GJB2 gene (which encodes the protein connexin 26), and the mutations are usually recessive. So, two parents with one mutation each can have normal hearing. But if their child gets two faulty copies of the gene, the child will have a hearing loss. In fact, the majority of children born with hearing loss have normal hearing parents.
There are limits to genetic testing, however. For one thing not all the genes are known—yet. Also, a positive result for a mutation does not necessarily mean a person will get the condition associated with the mutation. And a negative result doesn’t mean you won’t get the particular condition, too—it may be that a different mutation in the same gene wasn’t detected, or there could be another mutation in a different gene that may cause the condition.
We have compiled a list of several dozen genetic testing centers nationwide that have specialized testing for hearing loss. Find a testing center near you.