Because they are relatively basic, over-the-counter hearing aids may end up pushing features like directional hearing and telecoils into prescription hearing aids for more severe types of hearing loss.
The Connection Between Hearing Loss and Dementia
By Alycia Gordan
June is Alzheimer's & Brain Awareness Month and Hearing Health Foundation would like to shine light on the effects untreated hearing loss can have on our brains and memory. Hearing loss is often linked with dementia, and research is being conducted to establish the exact link between the two. Evidence suggests that by treating hearing loss, the risk of dementia can be mitigated.
Dementia is a medical term that is used to describe a host of symptoms, characterized by a deterioration in a patient’s cognitive abilities. The degeneration of brain cells causes neurons to stop functioning, leading to a series of dysfunctions.
A person may have dementia if at least two of his mental faculties are affected: the loss of memory and focus; difficulty communicating; short or interrupted attention spans; impaired judgment; or an inability to perform everyday tasks.
Frank Lin, M.D., Ph.D., an associate professor of otolaryngology and epidemiology at Johns Hopkins University, conducted a study in 2011 in which the mental abilities of 639 cognitively stable individuals were supervised regularly for 12 to 18 years. The results indicated that volunteers with normal hearing were much less susceptible to acquiring dementia while those with mild, moderate, and severe hearing loss were two, three, and five times more susceptible to the disorder, respectively.
Another study conducted by Lin in 2013 involved observing the cognitive abilities of 1,984 older adults over six years. The research concluded that older adults with hearing loss tended to experience 30 to 40 percent accelerated cognitive dysfunction and were at a higher risk of developing dementia.
What Is the Cause?
Since the exact link between hearing loss and dementia is still a mystery, there are theories about how the former may aggravate the latter.
One of the theories suggests that if the brain struggles to cope with degraded sounds, its resources are allocated to processing these sounds and this “cognitive load” causes a decrease in overall cognitive functioning. Moreover, hearing loss accelerates atrophy in the cerebrum which is not exclusive to processing sound as it also plays a role in memory. In addition, it is speculated that social isolation that results from hearing loss causes stress and depression and exacerbates cognitive deterioration.
What Is the Solution?
Not many studies have been conducted to check the influence of treating hearing loss for treating dementia. However, the studies that have been conducted so far do provide considerable hope.
One way to improve profound hearing loss is receiving cochlear implants. French researcher Isabelle Mosnier, M.D., of the Assistance Publique-Hôpitaux de Paris, evaluated the effect of cochlear implants on cognitive functioning in 94 elderly people who had profound deafness (in at least one ear).
Mosnier found that hearing rehabilitation improved not only cognitive functioning of the elderly, but their speech perception as well.
The most direct link between auditory impairment and memory loss is the brain. Thus, any stimulus that helps the brain remain alert will keep the person active too. Hence, researchers are considering the use of music therapy to restore cognitive functions in people who suffer from memory loss.
Concetta Tomaino, a cofounder of the Institute for Music and Neurological Function, found that music stimulates parts of the brain made inactive by dementia. In a pilot study, music therapy sessions were conducted with 45 individuals with chronic dementia and the results showed that neurological and cognitive abilities improved significantly for those in the music group.
This research shows there are techniques that can aid individuals with dementia and hearing loss. If you or a loved one has hearing problems, please see a hearing health professional to get a hearing test in order to potentially prevent future cognitive issues.
Alycia Gordan writes for Brain Blog.
Second Cause of Hidden Hearing Loss Identified
By Michigan Medicine - University of Michigan
Some people can pass a hearing test but have trouble understanding speech in a noisy environment. New research identifies a new mechanism for this condition just years after its discovery. Credit: Michigan Medicine
Patients who complain they can't hear their friends at a noisy restaurant, but pass a hearing test in their doctor's office, may be describing hidden hearing loss.
Now, less than six years since its initial description, scientists have made great strides in understanding what hidden hearing loss is and what causes it. In research published in Nature Communications, University of Michigan researchers report a new unexpected cause for this auditory neuropathy, a step toward the eventual work to identify treatments.
"If people can have hidden hearing loss for different reasons, having the ability to make the right diagnosis of the pathogenesis will be critical," says author Gabriel Corfas, Ph.D., director of the Kresge Hearing Research Institute at Michigan Medicine's Department of Otolaryngology -- Head and Neck Surgery.
Corfas published the research with co-author Guoqiang Wan, now with Nanjing University in China. They discovered using mice that disruption in the Schwann cells that make myelin, which insulates the neuronal axons in the ear, leads to hidden hearing loss. This means hidden hearing loss could be behind auditory deficits seen in acute demyelinating disorders such as Guillain-Barré syndrome, which can be caused by Zika virus.
Corfas and Wan used genetic tools to induce loss of myelin in the auditory nerve of mice, modeling Guillain-Barré. Although the myelin regenerated in a few weeks, the mice developed a permanent hidden hearing loss. Even after the myelin regenerated, damage to a nerve structure called the heminode remained.
Synapse loss versus myelin disruption
When the ear is exposed to loud noises over time, synapses connecting hair cells with the neurons in the inner ear are lost. This loss of synapses has previously been shown as a mechanism leading to hidden hearing loss.
In an audiologist's quiet testing room, only a few synapses are needed to pick up sounds. But in a noisy environment, the ear must activate specific synapses. If they aren't all there, it's difficult for people to make sense of the noise or words around them. That is hidden hearing loss, Corfas says.
"Exposure to noise is increasing in our society, and children are exposing themselves to high levels of noise very early in life," Corfas says. "It's clear that being exposed to high levels of sound might contribute to increases in hidden hearing loss."
The newly identified cause -- deficiency in Schwann cells -- could occur in individuals who have already had noise exposure-driven hidden hearing loss as well. "Both forms of hidden hearing loss, noise exposure and loss of myelin, can occur in the same individual for an additive effect," Corfas says.
Previously, Corfas' group succeeded in regenerating synapses in mice with hidden hearing loss, providing a path to explore for potential treatment.
While continuing this work, Corfas started to investigate other cells in the ear, which led to uncovering the new mechanism.
There are no current treatments for hidden hearing loss. But as understanding of the condition improves, the goal is for the research to lead to the development of drugs to treat it.
"Our findings should influence the way hidden hearing loss is diagnosed and drive the future of clinical trials searching for a treatment," Corfas says. "The first step is to know whether a person's hidden hearing loss is due to synapse loss or myelin/heminode damage."
Materials provided by Michigan Medicine - University of Michigan. Co-author Guoqiang Wan, Ph.D., was a 2014 Emerging Research Grants recipient funded by the Wes Bradley, M.D. Memorial Grant.
We need your help in funding the exciting work of hearing and balance scientists.
To donate today to support HHF's groundbreaking research, please visit hhf.org/donate.
A Fight Against Cancer Is a Fight Against Hearing Loss
By Frankie Huang
In honor of World Cancer Day on February 4, Hearing Health Foundation (HHF) wants to raise awareness of the connection between cancer and hearing loss. Every year, 8.2 million people worldwide die from cancer, a disease that is responsible for 13% of all deaths globally.
Depending on the type of cancer, patients that undergo chemotherapy are sometimes required to take certain drugs that could cause many side effects, including hearing loss. Cisplatin is a chemotherapy drug that is often used to treat testicular, bladder, ovarian and lung cancers. However, an excessive dose of cisplatin can be ototoxic (toxic to the ear), which could lead to temporary or permanent hearing loss.
One study suggested that cisplatin-induced hearing loss is generally bilateral (both ears) and irreversible. The study also found that cisplatin accumulates in cochlear tissue, preventing the cochlea from flushing out toxins. The same researchers found that patients receiving doses of cisplatin between 150-225 mg/m2 showed some degree of hearing loss. For testicular cancer patients, more than 50% of the patients that took cisplatin in doses greater than 400 mg/m2 had permanent hearing loss. Hearing loss may occur within hours or days after the treatment, or hearing may gradually decline after completion of therapy. After following up more than two years later, the study authors found that 44% of patients who took cisplatin had significant hearing loss.
In another recent study, researchers found that the WFS1 gene is associated with cisplatin-related ototoxicity; the heavier the dose, the more severe the hearing loss. Also, a mutation of the WFS1 gene results in Wolfram syndrome, a disorder with deafness as a major symptom.
As of now, there are no safe and protective agents against cisplatin, but scientists are hard at work to find a protective agent that would eliminate the negative side effects of cisplatin. Currently there’s a solution for children that are receiving cisplatin-based chemotherapy: The use of sodium thiosulfate may minimize or protect children and adolescents against cisplatin-induced hearing loss. HHF hopes more preventative therapies and cures for hearing loss can be discovered for all cisplatin-treated patients.
Interested in funding research in this area? Email us at development@hhf.org.
Scientists restore hearing in noise-deafened mice
By the University of Michigan Health System
Scientists have restored the hearing of mice partly deafened by noise, using advanced tools to boost the production of a key protein in their ears.
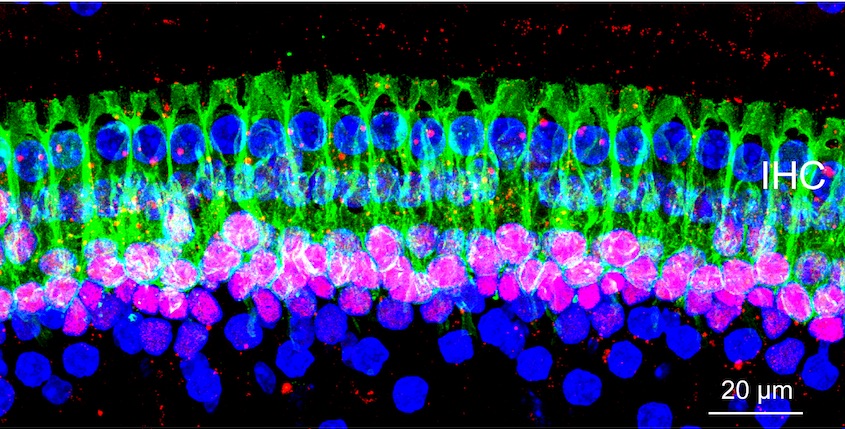
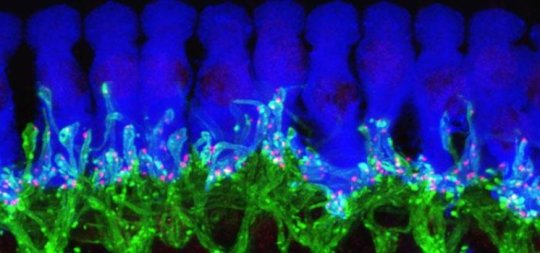
This microscope image of tissue from deep inside a normal mouse ear shows how ribbon synapses (red) form the connections between the hair cells of the inner ear (blue) and the tips of nerve cells (green) that connect to the brain.
Credit: Corfas lab - University of Michigan
By demonstrating the importance of the protein, called NT3, in maintaining communication between the ears and brain, these new findings pave the way for research in humans that could improve treatment of hearing loss caused by noise exposure and normal aging.
In a new paper in the online journal eLife, the team from the University of Michigan Medical School's Kresge Hearing Research Institute and Harvard University report the results of their work to understand NT3's role in the inner ear, and the impact of increased NT3 production on hearing after a noise exposure.
Their work also illustrates the key role of cells that have traditionally been seen as the "supporting actors" of the ear-brain connection. Called supporting cells, they form a physical base for the hearing system's "stars": the hair cells in the ear that interact directly with the nerves that carry sound signals to the brain. This new research identifies the critical role of these supporting cells along with the NT3 molecules that they produce.
NT3 is crucial to the body's ability to form and maintain connections between hair cells and nerve cells, the researchers demonstrate. This special type of connection, called a ribbon synapse, allows extra-rapid communication of signals that travel back and forth across tiny gaps between the two types of cells.
"It has become apparent that hearing loss due to damaged ribbon synapses is a very common and challenging problem, whether it's due to noise or normal aging," says Gabriel Corfas, Ph.D., who led the team and directs the U-M institute. "We began this work 15 years ago to answer very basic questions about the inner ear, and now we have been able to restore hearing after partial deafening with noise, a common problem for people. It's very exciting."
Using a special genetic technique, the researchers made it possible for some mice to produce additional NT3 in cells of specific areas of the inner ear after they were exposed to noise loud enough to reduce hearing. Mice with extra NT3 regained their ability to hear much better than the control mice.
Now, says Corfas, his team will explore the role of NT3 in human ears, and seek drugs that might boost NT3 action or production. While the use of such drugs in humans could be several years away, the new discovery gives them a specific target to pursue.
Corfas, a professor and associate chair in the U-M Department of Otolaryngology, worked on the research with first author Guoqiang Wan, Ph.D., Maria E. Gómez-Casati, Ph.D., and others in his former institution, Harvard. Some of the authors now work with Corfas in his new U-M lab. They set out to find out how ribbon synapses -- which are found only in the ear and eye -- form, and what molecules are important to their formation and maintenance.
Anyone who has experienced problems making out the voice of the person next to them in a crowded room has felt the effects of reduced ribbon synapses. So has anyone who has experienced temporary reduction in hearing after going to a loud concert. The damage caused by noise -- over a lifetime or just one evening -- reduces the ability of hair cells to talk to the brain via ribbon synapse connections with nerve cells.
Targeted genetics made discovery possible
After determining that inner ear supporting cells supply NT3, the team turned to a technique called conditional gene recombination to see what would happen if they boosted NT3 production by the supporting cells. The approach allows scientists to activate genes in specific cells, by giving a dose of a drug that triggers the cell to "read" extra copies of a gene that had been inserted into them. For this research, the scientists activated the extra NT3 genes only into the inner ear's supporting cells.
The genes didn't turn on until the scientists wanted them to -- either before or after they exposed the mice to loud noises. The scientists turned on the NT3 genes by giving a dose of the drug tamoxifen, which triggered the supporting cells to make more of the protein. Before and after this step, they tested the mice's hearing using an approach called auditory brainstem response or ABR -- the same test used on humans.
The result: the mice with extra NT3 regained their hearing over a period of two weeks, and were able to hear much better than mice without the extra NT3 production. The scientists also did the same with another nerve cell growth factor, or neurotrophin, called BDNF, but did not see the same effect on hearing.
Next steps
Now that NT3's role in making and maintaining ribbon synapses has become clear, Corfas says the next challenge is to study it in human ears, and to look for drugs that can work like NT3 does. Corfas has some drug candidates in mind, and hopes to partner with industry to look for others.
Boosting NT3 production through gene therapy in humans could also be an option, he says, but a drug-based approach would be simpler and could be administered as long as it takes to restore hearing.
Corfas notes that the mice in the study were not completely deafened, so it's not yet known if boosting NT3 activity could restore hearing that has been entirely lost. He also notes that the research may have implications for other diseases in which nerve cell connections are lost -- called neurodegenerative diseases. "This brings supporting cells into the spotlight, and starts to show how much they contribute to plasticity, development and maintenance of neural connections," he says.
In addition to Corfas, Wan and Gómez-Casati, who now works in Argentina, the research was performed by Angelica R. Gigliello, and M. Charles Liberman, Ph.D. director of the Eaton-Peabody Laboratories of the Massachusetts Eye and Ear Infirmary. The research was supported by the National Institute on Deafness and Other Communication Disorders (DC004820, DC005209) and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD18655), both part of the National Institutes of Health, and by the Hearing Health Foundation.
The above post is reprinted from materials provided by University of Michigan Health System.
We need your help in funding the exciting work of hearing and balance scientists.
To donate today to Hearing Health Foundation and support groundbreaking research, visit hhf.org/name-a-grant.
The Case for Genetic Testing
By Yishane Lee
Genetic causes account for roughly half of hearing loss cases in infants, according to the Centers for Disease Control and Prevention. Many cases of progressive hearing loss that occur after infancy and childhood also have a genetic component.
At least 1,000 mutations in 64 genes linked to hearing loss have been identified. Thanks to rapid advances in genetic sequencing, identifying virtually all the genetic causes of hearing loss may occur within the decade, according to a recent report in the journal Genetic Testing and Molecular Biomarkers.
Researchers are using “targeted resequencing” to locate gene mutations in certain regions in the human genome that are linked to diseases much more quickly than sequencing the entire genome. In our Fall 2013 issue of Hearing Health magazine, Xue Zhong Liu, M.D., Ph.D., reviews the advances in sequencing technology and how this will affect the future treatment of hearing loss.
Because genetics can play such a significant role in hearing loss, genetic testing can answer questions you have about the cause of your or a loved one’s hearing loss. If the testing uncovers a mutation, it can help explain the hearing loss, its severity or progression, and whether other symptoms may become apparent. For instance, a person with Usher syndrome has not only hearing loss but also eventual blindness. You can proactively take steps to manage treatment and outcome. Knowing the genetic cause of a hearing loss can also help you predict whether the condition will be passed along to your children, or whether the children of other family members may have the condition.
Last summer, Hearing Health magazine presented an overview of genetic causes of hearing loss, including Connexin 26 disorder. This is the most common cause of congenital hearing loss not related to a syndrome (with other symptoms, such as a thyroid problem). Mutations in the GJB2 gene affect development of the cochlea in the inner ear. Everyone carries two copies of the GJB2 gene (which encodes the protein connexin 26), and the mutations are usually recessive. So, two parents with one mutation each can have normal hearing. But if their child gets two faulty copies of the gene, the child will have a hearing loss. In fact, the majority of children born with hearing loss have normal hearing parents.
There are limits to genetic testing, however. For one thing not all the genes are known—yet. Also, a positive result for a mutation does not necessarily mean a person will get the condition associated with the mutation. And a negative result doesn’t mean you won’t get the particular condition, too—it may be that a different mutation in the same gene wasn’t detected, or there could be another mutation in a different gene that may cause the condition.
We have compiled a list of several dozen genetic testing centers nationwide that have specialized testing for hearing loss. Find a testing center near you.
Research on Cell Regeneration Highlighted in the New York Times
Two research reports published Friday offer novel approaches to the age-old dream of regenerating the body from its own cells.
Animals like newts and zebra fish can regenerate limbs, fins, even part of the heart. If only people could do the same, amputees might grow new limbs and stricken hearts be coaxed to repair themselves.
But humans have very little regenerative capacity, probably because of an evolutionary trade-off: suppressing cell growth reduced the risk of cancer, enabling humans to live longer. A person can renew his liver to some extent, and regrow a fingertip while very young, but not much more.
In the first of the two new approaches, a research group at Stanford University led by Helen M. Blau, Jason H. Pomerantz and Kostandin V. Pajcini has taken a possible first step toward unlocking the human ability to regenerate. By inactivating two genes that work to suppress tumors, they got mouse muscle cells to revert to a younger state, start dividing and help repair tissue.