Ver pagina en: English | Español
Hearing is complex, requiring a series of actions and reactions to work. The process involves many parts of the ear working together to convert sound waves into information the brain understands and interprets.
Sound waves enter the ear canal and travel toward our eardrums.
The sound waves cause the eardrum and bones in the middle ear to vibrate.
Tiny hair cells inside the cochlea, the sensory organ of the ear, convert these vibrations into electric impulses that are picked up by the auditory nerve.
At birth, each typical ear has about 12,000 sensory cells, called hair cells, which sit on a membrane that vibrates in response to incoming sound. Each frequency of a complex sound maximally vibrates the membrane at one location. Because of this mechanism, we hear different pitches within the sound. A louder sound increases the amplitude of the vibration, so we hear loudness.
Signals sent to the brain from auditory nerve are then interpreted as sounds.
Once the hair cells in the inner ear are damaged, permanent sensorineural hearing loss occurs.
Currently, sensorineural hearing loss cannot be restored in humans, but HHF’s researchers are working to better understand the mechanisms of hearing loss to find better treatments and cures.
More Resources
For individuals with long-term hearing loss or severely degraded auditory input, the lack of reliable auditory feedback represents a challenge many orders of magnitude greater than the temporary masking used in this study.
It bears repeating: What improves access for a group with a specific disability invariably also helps the greater population.
Because noise-canceling earbuds are so comfortable and block everything out, people wear them for three, four, five hours straight without realizing the cumulative effect on their ears.
I made one hat to solve problems, never imagining how many other adults and children would relate. It’s an honor to be able to give something back to the cochlear implant community that understands this journey so well.
These findings suggest that the ability to integrate what is seen with what is heard becomes increasingly important with age, especially for cochlear implant users.
Tinnitus Quest’s Tinnitus Hackathon prioritized active problem-solving, cross-disciplinary debate, and the development of a shared research agenda.
As the first known Black author to publish a 10-book children’s series centered on deaf, hard of hearing, and disabled heroes, I’ve created what I once longed for: stories where children see themselves as powerful.
Social platforms have become spaces to compare symptoms, crowdsource explanations, and seek community. For tinnitus, that openness has helped many people feel less alone. Unfortunately, it has also created space for confusion, misinformation, and discouraging myths that can delay effective care.
Often these surprising sources of loud sounds come about from a misguided belief that loud means fun—the louder it is, the more festive. The good news? Because the decibel scale is logarithmic, turning it down even a little can help save our hearing a lot.
When thinking about exposure to loud sounds, it is important to take a life-course perspective. That is, the health behaviors developed in childhood and adolescence can shape habits into adulthood.




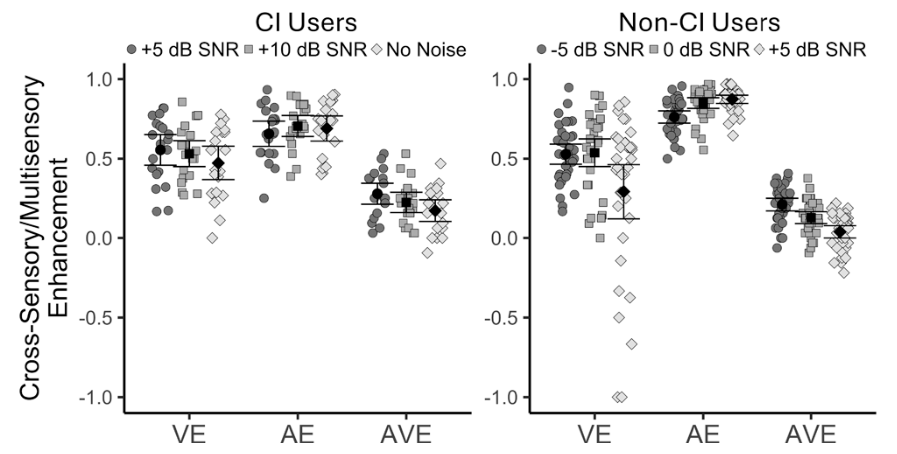




We are proud that Hearing Health Foundation-funded scientists are always well represented at Association for Research in Otolaryngology MidWinter Meeting.