John Oghalai, M.D. (a 1996–97 ERG scientist), of the University of Southern California, coauthored a May 7, 2018, study in the Proceedings of the National Academy of Sciences showing promise for preventing noise-induced hearing loss. Using a mouse model, the investigators found that in addition to immediate hair cell death after loud noise exposure, a fluid buildup in the inner ear occurs, eventually leading to nerve cell loss. Because the extra fluid shows a high potassium level, the researchers saw a method to rebalance the fluid by injecting a salt and sugar solution into the ear. Nerve cell loss was reduced by 45 to 64 percent, which the team says may preserve hearing. The team sees future applications for military service members exposed to blast trauma and patients with the hearing and balance disorder Ménière’s disease. —Y.L.
Novel Drug-Delivery Method to the Inner Ear
By Gary Polakovic, USC News
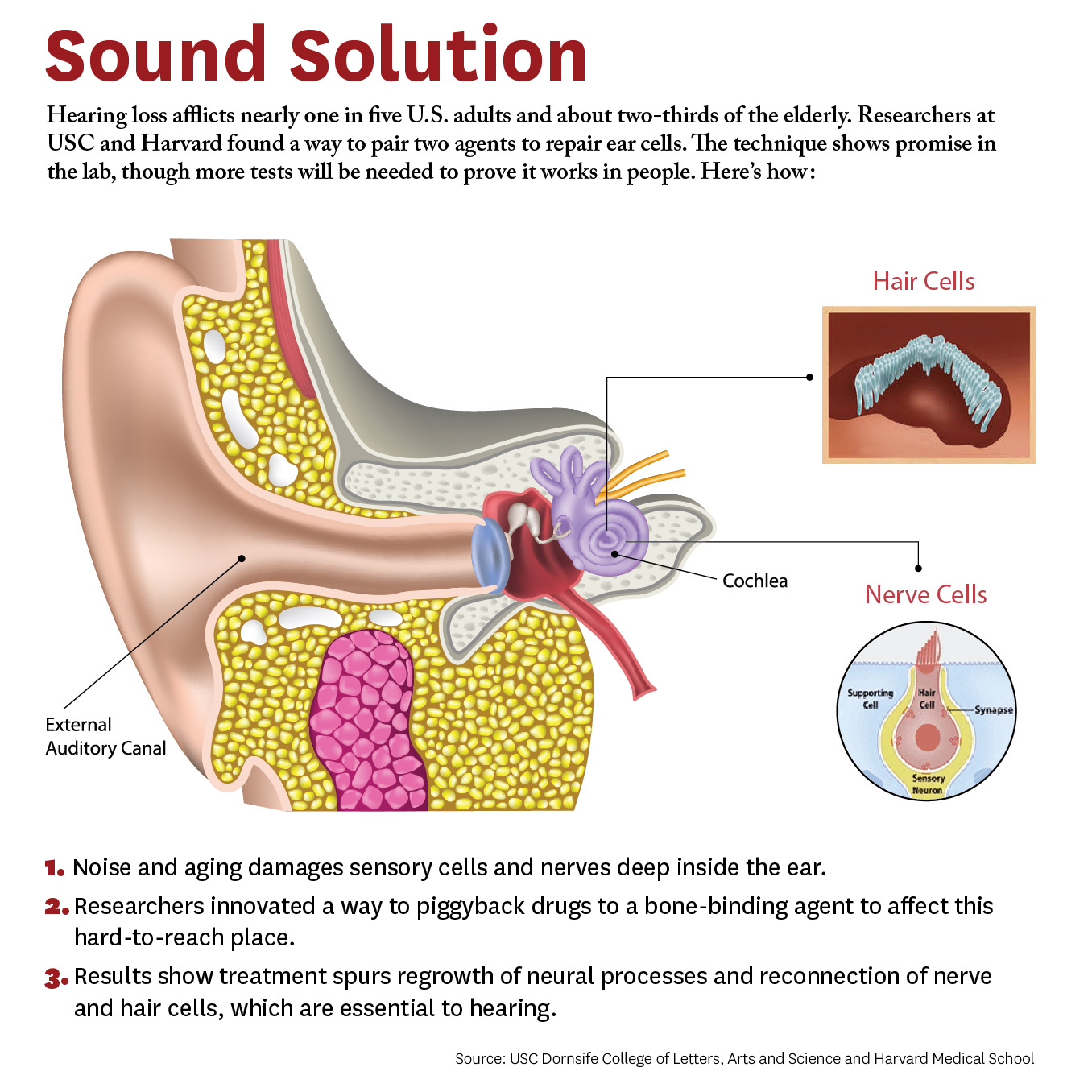
Researchers have developed a new approach to be able to repair cells deep inside the ear. The study, conducted by scientists at University of Southern California (USC) and Harvard University, demonstrates a novel way for a future drug to zero in on damaged nerves and cells inside the ear.
Credit: Matthew Pla Savino/USC News
“What’s new here is we figured out how to deliver a drug into the inner ear so it actually stays put and does what it’s supposed to do, and that’s novel,” says Charles E. McKenna, Ph.D., a corresponding author for the study and chemistry professor at the USC Dornsife College of Letters, Arts, and Sciences.
“Inside this part of the ear, there’s fluid constantly flowing that would sweep dissolved drugs away, but our new approach addresses that problem. This is a first for hearing loss and the ear,” McKenna adds. “It’s also important because it may be adaptable for other drugs that need to be applied within the inner ear.”
The paper was published April 4 in the journal Bioconjugate Chemistry. The authors include lead researcher Judith S. Kempfle, Ph.D., a 2011 and 2012 Emerging Research Grants scientist, as well as Hearing Restoration Project member Albert Edge, Ph.D., both at Harvard Medical School and The Eaton-Peabody Laboratories in Boston.
There are caveats. The research was conducted on animal tissues in a petri dish. It has not yet been tested in living animals or humans. Yet the researchers are hopeful given the similarities of cells and mechanisms involved. McKenna says since the technique works in the laboratory, the findings provide “strong preliminary evidence” it could work in living creatures. They are already planning the next phase involving animals and hearing loss.
The study breaks new ground because researchers developed a novel drug-delivery method. Specifically, it targets the cochlea, a snail-like structure in the inner ear where sensitive cells convey sound to the brain. Hearing loss occurs due to aging or exposure to noise at unsafe levels. Over time, hair-like sensory cells and bundles of neurons that transmit their vibrations break down, as do ribbon-like synapses, which connect the cells.
The researchers designed a molecule combining 7,8-dihydroxyflavone, which mimics a protein critical for development and function of the nervous system, and bisphosphonate, a type of drug that sticks to bones. This pairing delivered the breakthrough solution, the researchers say, as neurons responded to the molecule and regenerated synapses in mouse ear tissue. This led to the repair of the hair cells and neurons, which are essential to hearing.
“We’re not saying it’s a cure for hearing loss,” McKenna says. “It’s a proof of principle for a new approach that’s extremely promising. It’s an important step that offers a lot of hope.” Hearing loss affects two thirds of people over 70 years and 17 percent of all adults in the United States, and it is expected to nearly double in 40 years.
This is adapted from "Hearing Loss Study at USC, Harvard Shows Hope for Millions" on the USC News website. The authors of the April 4, 2018, Bioconjugate Chemistry study, “Bisphosphonate-Linked TrkB Agonist: Cochlea-Targeted Delivery of a Neurotrophic Agent as a Strategy for the Treatment of Hearing Loss,” include lead researcher Judith S. Kempfle, as well as Christine Hamadani, Nicholas Koen, Albert S. Edge, and David H. Jung of Harvard Medical School and The Eaton-Peabody Laboratories/Massachusetts Eye and Ear in Boston. Kempfle is also affiliated with the University of Tübingen Medical Center. Corresponding author Charles E. McKenna, as well as Kim Nguyen and Boris A. Kashemirov, are at USC Dornsife.
The research was supported by the American Academy of Otolaryngology–Head and Neck Surgery Herbert Silverstein Otology and Neurotology Research Award, an American Otological Society Research Grant, and a grant from the National Institute of Deafness and other Communicative Disorders (R01 DC007174).
Cochlear to Support Hearing Research By Reaching One Million Ears
Today marks the start of Better Hearing & Speech Month (BHSM), a campaign to advance public knowledge of communication disorders. To celebrate, international hearing implant manufacturer Cochlear is launching the #MillionEar Challenge with the goal of informing one million people about the importance of hearing health and research.
Proceeds from the campaign will benefit Hearing Health Foundation (HHF)’s longstanding Emerging Research Grants (ERG) program. Cochlear has pledged to donate to ERG when the #MillionEar Challenge is met.
“Awareness is at the heart of Hearing Health Foundation's efforts to prevent, treat, and cure hearing loss," said Nadine Dehgan, HHF’s Chief Executive Officer. "I am deeply grateful Cochlear is committed to raising awareness of hearing loss, which will inspire more to pursue hearing tests and life-changing treatments."
HHF staff thanks Cochlear in their own #MillionEar Challenge shirts.
Cochlear’s generous gift will allow HHF to continue funding up-and-coming scientists who investigate various hearing and balance conditions. Such funding has historically led to the development of many new treatments including cochlear implants which, today, benefit more than 300,000 people worldwide.
You can support the #MillionEar campaign with the purchase of a t-shirt, available in child and adult sizes. Read the full press from Cochlear release here.
Designing New Antibiotics That Aren't Harmful to Hearing
Anthony Ricci, Ph.D. (1999–2000 ERG), a professor of otolaryngology–head and neck surgery at Stanford University, and Alan Cheng, M.D. (2002–03 ERG), a Stanford associate professor of otolaryngology, are developing a new type of aminoglycosides, a widely used, life-saving class of antibiotic that fights a broad range of serious infections and diseases such as cystic fibrosis and tuberculosis, but that also has the side effect of hearing loss in one in five patients. The pair have been collaborating since 2008, leveraging Ricci’s knowledge of mechanotransduction (how sound wave vibrations are converted into electrical signals) and ion channels. Of the 18 potential replacement antibiotics they created, three show the most promise for preserving hearing while remaining effective in killing bacteria and will be tested further. —Y.L.
Tony Ricci, left, and Alan Cheng have built a team to develop a safe replacement for a type of antibiotic that can cause deafness. The researchers use a patch clamp rig in Ricci’s lab to see which forms of the drug can enter Hair cells — cells crucial for hearing. Max Aguilera-Hellweg photography.
Is It Overstimulation?
By Eric Sherman
My younger son Cole has been wearing cochlear implants (CI) since 2005. He was barely a toddler, between 18 and 24 months old, when he rejected them.
The initial response from our audiologist was, “We just mapped your son, just do your best to keep the processor on his head.” Unique to every CI wearer, mapping adjusts the sound input to the electrodes on the array implanted into the cochlea. It is meant to optimize the CI user’s access to sound.
But after several weeks, and our audio-verbal therapist told us there was something wrong and referred us to another pediatric audiologist, Joan Hewitt, Au.D.
Eric Sherman and his son, Cole
We learned that refusing to wear CI processors is generally a symptom of a problem that a child can’t necessarily express. Their behavior becomes the only way to communicate the issue.
“Our brains crave hearing,” Hewitt says. “Children should want to have their CIs on all the time. If a child resists putting the CIs on in the morning, cries or winces when they are put on, or fails to replace the headpiece when it falls off, there is a strong possibility that the CIs are providing too much stimulation. Some children appear shy or withdrawn because the stimulation is so great that interacting is painful. Others respond to overstimulation by being loud and aggressive.”
Hewitt says research discussed at the Cochlear Implant Symposium in Chicago in 2011 (or CI2011, run by the then-newly created American Cochlear Implant Alliance) addressed the issue of overstimulation. A study that was presented, titled "Overstimulation in Children with Cochlear Implants," listed symptoms that indicated children were overstimulated by their cochlear implants: reluctance or refusal to wear the device, overly loud voices, poor articulation, short attention span or agitated behavior, and no improvement in symptoms despite appropriate therapy.
When the researchers reduced the stimulation levels, they found very rapid improvement in voice quality and vocal loudness and gradual improvement in articulation. Finally, they found “surprising effects on the children's behavior”—the parents reported a marked improvement in attention and reduction in agitation.
In “Cochlear Implants—Considerations in Programming for the Pediatric Population,” in AudiologyOnline, Jennifer Mertes, Au.D., CCC-A, and Jill Chinnici, CCC-A, write: “Children are not little adults. They are indeed, unique, and to address their CI needs, they require an experienced clinician. Most children are unable to provide accurate feedback while the audiologist programs their cochlear implant and therefore, the clinician must take many things into account.”
These include:
The audiologists' past experiences with other patients
Updated information regarding the child's progress (from parents, therapists, and teachers
Audiometric test measures
Observations of the child during programming
Objective measurements
If age appropriate, the clinician will train the child to participate in programming
Many of the decisions made during programming appointments come from the clinician's knowledge and experience, rather than the child's behavioral responses. But your child’s reactions should also be taken into account.
If your child continues to refuse to wear their processors after a remapping, take into consideration your audiologist’s experience and mapping approach and seek a second opinion. When we met with Hewitt, she found our child’s map was overstimulating. Once she remapped using a different approach, our son had no problem wearing his CI processor again.
Los Angeles marketing executive Eric Sherman is the founder of Ci Wear, a patented shirt designed to secure and protect cochlear implant processors. April is National Autism Awareness Month. Read about how Sherman and Cole manage Cole’s hearing loss and autism spectrum disorder conditions in “When It’s Not Just Hearing Loss” in the Fall 2016 issue of Hearing Health.
Let’s Make Noise Safer
By Vicky Chan
April 25 is International Noise Awareness Day, an annual, vital reminder to take a stand against noise exposure and to spread awareness about the underestimated threat of noise-induced hearing loss (NIHL). Seemingly harmless rhythms, roars, and blasts heard daily from music, trains, and machinery are, in fact, among the top offenders of NIHL.
Noise-induced hearing loss (NIHL) progressively occurs after chronic exposure to loud sounds. The frequency and intensity of the sound level, measured in decibels (dB), increases the risk of NIHL. Gradual hearing loss can result from prolonged contact with noise levels of 85 dB or greater, such as heavy city traffic. Noises of 110 dB or more, like construction (110 dB), an ambulance (120dB), or the pop of firecrackers (140-165 dB) can damage one’s hearing in a minute’s time.
NIHL is the only type of hearing loss that is completely preventable, yet billions of individuals endanger themselves daily. Over 1.1 billion young adults ages 12 to 35—an age group that frequently uses headphones to listen to music—are at risk. Already, an estimated 12.5% of young people ages of 6 to 19 have hearing loss as a result of using earbuds or headphones at a high volume. A device playing at maximum volume (105 dB) is dangerous, so exposure to sounds at 100 dB for more than 15 minutes is highly discouraged.
Most major cities around the world have transit systems that put commuters in contact with sounds at 110 dB. BBC News found that London’s transit systems can get as loud as 110 dB, which is louder than a nearby helicopter taking off. The sound levels of some stations exceed the threshold for which occupational hearing protection is legally required. New York City has one of the largest and oldest subway systems in the world where 91% of commuters exceed the recommended levels of noise exposure annually. In a study on Toronto’s subway system, 20% of intermittent bursts of impulse noises were greater than 114 dB.
People who work in certain fields are more vulnerable to NIHL than others. Professional musicians, for instance, are almost four times as likely to develop NIHL than the general public. Military personnel, who are in extremely close proximity to gunfire and blasts, are more likely to return home from combat with hearing loss and/or tinnitus than any other type of injury. And airport ground staff are surrounded by high-frequency aircraft noises at 140 dB. In all of these professions, the hazard of NIHL can be significantly mitigated with hearing protection.
NIHL is permanent. Increased exposure to excess noise destroys the sensory cells in the inner ears (hair cells), which decreases hearing capacity and leads to hearing loss. Once damaged, the sensory cells cannot be restored. To find a solution, Hearing Health Foundation’s (HHF) Hearing Restoration Project (HRP) conducts groundbreaking research on inner ear hair cell regeneration in hopes of discovering a life-changing cure.
Nearly three-quarters of those who are exposed to loud noises rarely or never use hearing protection. It is our dream that someday, NIHL will be reversible as a result of the HRP. Until then, to make noise safer, HHF advises protection by remembering to Block, Walk, and Turn. Block out noises by wearing earplugs or protective earmuffs. Walk away or limit exposure to high-levels of noises. Turn down the volume of electronic devices.
Sound Processing in Early Brain Regions
To better identify and treat these central auditory processing disorders that appear despite normal ear function, 2016 Emerging Research Grants (ERG) scientist Richard A. Felix II, Ph.D., and colleagues have been investigating how the brain processes complex sounds such as speech.
Teaching on a Different Route
By Lauren McGrath
Assistant Teacher Ms. Tiana Brown with two of her preschool students at Clarke.
The clock moves toward 9:00 AM as two teachers oversee the listening check with their preschool students, ages four to five, to verify that their hearing devices are operating properly. A critical test for children with hearing loss, the check is step one each day for colleagues Ms. Kathryn Smith, Teacher of the Deaf, and Ms. Tiana Brown, Assistant Teacher at Clarke Schools for Hearing and Speech in New York.
Assured that all devices allow optimal access to sound, Ms. Kathryn and Ms. Tiana are ready to begin a busy day in the classroom. Beyond following a typical preschool curriculum with pre-reading, pre-academics, math, science, art, music, and language, the two teachers lead social and emotional development and self-help instruction. Throughout the day, Ms. Kathryn and Ms. Tiana track students’ progress toward goals they've defined as part of each child’s professional team. Each team is comprised of a unique set of professionals, based on individual students' strengths and needs.
Both Ms. Kathryn and Ms. Tiana have long been passionate about working with children. Ms. Tiana takes pride in being an advocate who can provide emotional support to kids and Ms. Kathryn feels fortunate to spend her career working with young people who are full of wonder and excitement.
Ms. Kathryn Smith, Teacher of the Deaf, smiles with a student.
Ms. Kathryn holds a Bachelor's in Communication Disorders with a minor in Deaf Studies from SUNY New Paltz and a Master’s in Deaf Education from Hunter College. Ms. Tiana completed her Bachelor’s in Communication Disorders at St. John’s University. After developing interests in aural rehabilitation in school, working with children who are deaf or hard-of-hearing—where they can contribute to the success of many children with unique perspectives and experiences—was a natural career choice for both Ms. Kathryn and Ms. Tiana.
The progress that Clarke students make, despite not having the same abilities as their typical-hearing peers, impresses the teachers. Though the children have an “added challenge at the starting line,” they experience tremendous growth as a result of their efforts made both independently and in collaboration with their families and professionals, says Ms. Kathryn. She recalls a few of her classroom’s latest accomplishments. One child is celebrating her newfound ability to put her FM system on all by herself. Another student who recently received a cochlear implant is regularly responsive to the sound of his name in the noisy classroom.
Ms. Tiana reflects on positive experiences outside the classroom, such as daily trips to the park, which she particularly enjoys. “As soon as we step outside, a whole new world opens up for them. They tell me about the sounds they hear and the sights they observe—and I know they’re not missing out on a single piece of life.” She feels most rewarded at work when a student expresses gratitude for help she provided.
At 2:30 PM, the Clarke students make their way out of school and home to their families. As staff, Ms. Kathryn and Ms. Tiana also build relationships with the school’s families who, like the students, greatly admire the teachers and look to them for guidance. Ms. Kathryn reminds parents and families not to lose sight of their child in the diagnosis. “Your child has a hearing loss, but it is not all of them. Your hopes and dreams for your child can still be achieved; they may just take a different route.”
A Clinical Trial for a New Drug to Protect Hearing
By Yishane Lee
The U.S. Food and Drug Administration (FDA) has approved a novel drug to protect against ototoxicity (harmfulness to hearing) due to the use of aminoglycoside antibiotics to treat severe infections. The FDA approval paves the way for a Phase I clinical trial to test whether the drug, found to be significantly protective in animals, is safe for humans.
Mature lateral line hair cells from larval zebrafish (shown with the neuromast sensory organ enlarged) serve as a platform for studying drugs and genes that modulate hair cell susceptibility to ototoxic agents.
The drug, ORC-13661, was developed by University of Washington professors Edwin Rubel, Ph.D., and David Raible, Ph.D., who are members of Hearing Health Foundation’s Scientific Advisory Board and Hearing Restoration Project, respectively, and Fred Hutchinson Cancer Research Center scientist Julian Simons, Ph.D. “While this program was not directly funded by HHF, both David and I have definitely been supported by HHF for a long time,” Rubel says. “This is a drug to prevent hearing loss that we've developed over the past 15-plus years.”
Rubel points out the drug’s two main features: “It is a brand new drug with a composition of matter patent, not one that is used for other medical purposes and being repurposed; and it is the first drug that was developed, from the get-go, to protect hair cells from ototoxic injury.”
After screening libraries of potential chemicals to see which stopped hair cell death in zebrafish lateral line system, Rubel, Raible, and team identified the best candidate and then boosted its effectiveness by tweaking its chemical structure; results were published in the Journal of Medicinal Chemistry in January 2018.
Rubel adds, “Toxicity studies in zebrafish, rats, and dogs required by the FDA show superior safety and nearly 100 percent hearing protection at all frequencies.” If the Phase I trial shows the drug is safe for humans, the next step is to test its efficacy among patients using aminoglycosides.
New Data-Driven Analysis Procedure for Diagnostic Hearing Test
By Carol Stoll
Stimulus frequency otoacoustic emissions (SFOAEs) are sounds generated by the inner ear in response to a pure-tone stimulus. Hearing tests that measure SFOAEs are noninvasive and effective for those who are unable to participate, such as infants and young children. They also give valuable insight into cochlear function and can be used to diagnose specific types and causes of hearing loss. Though interpreting SFOAEs is simpler than other types of emissions, it is difficult to extract the SFOAEs from the same-frequency stimulus and from background noise caused by patient movement and microphone slippage in the ear canal.
2014 Emerging Research Grants (ERG) recipient Srikanta Mishra, Ph.D., and colleagues have addressed SFOAE analysis issues by developing an efficient data-driven analysis procedure. Their new method considers and rejects irrelevant background noise such as breathing, yawning, and subtle movements of the subject and/or microphone cable. The researchers used their new analysis procedure to characterize the standard features of SFOAEs in typical-hearing young adults and published their results in Hearing Research.
Mishra and team chose 50 typical-hearing young adults to participate in their study. Instead of using a discrete-tone procedure that measures SFOAEs one frequency at a time, they used a more efficient method: a single sweep-tone stimulus that seamlessly changes frequencies from 500 to 4,000 Hz, and vice versa, over 16 and 24 seconds. The sweep tones were interspersed with suppressor tones that reduce the response to the previous tone. The tester manually paused and restarted the sweep recording when they detected background noises from the subject’s movements.
The SFOAEs generated were analyzed using a mathematical model called a least square fit (LSF) and a series of algorithms based on statistical analysis of the data. This model objectively minimized the potential error from extraneous noises. Conventional SFOAE features such as level, noise floor, and signal-to-noise ratio (SNR) were described for the typical-hearing subjects.
Overall, the results of this study demonstrate the effectiveness of the automated noise rejection procedure of sweep-tone–evoked SFOAEs in adults. The features of SFOAEs characterized in this study from a large group of typical-hearing young adults should be useful for developing tests for cochlear function that can be useful in the clinic and laboratory.
Srikanta Mishra, Ph.D, was a 2014 Emerging Research Grants scientist and a General Grand Chapter Royal Arch Masons International award recipient. For more, see “Sweep-tone evoked stimulus frequency otoacoustic emissions in humans: Development of a noise-rejection algorithm and normative features” in Hearing Research.
We need your help supporting innovative hearing and balance science through our Emerging Research Grants program. Please make a contribution today.