By Gary Polakovic, USC News
Researchers have developed a new approach to be able to repair cells deep inside the ear. The study, conducted by scientists at University of Southern California (USC) and Harvard University, demonstrates a novel way for a future drug to zero in on damaged nerves and cells inside the ear.
Credit: Matthew Pla Savino/USC News
“What’s new here is we figured out how to deliver a drug into the inner ear so it actually stays put and does what it’s supposed to do, and that’s novel,” says Charles E. McKenna, Ph.D., a corresponding author for the study and chemistry professor at the USC Dornsife College of Letters, Arts, and Sciences.
“Inside this part of the ear, there’s fluid constantly flowing that would sweep dissolved drugs away, but our new approach addresses that problem. This is a first for hearing loss and the ear,” McKenna adds. “It’s also important because it may be adaptable for other drugs that need to be applied within the inner ear.”
The paper was published April 4 in the journal Bioconjugate Chemistry. The authors include lead researcher Judith S. Kempfle, Ph.D., a 2011 and 2012 Emerging Research Grants scientist, as well as Hearing Restoration Project member Albert Edge, Ph.D., both at Harvard Medical School and The Eaton-Peabody Laboratories in Boston.
There are caveats. The research was conducted on animal tissues in a petri dish. It has not yet been tested in living animals or humans. Yet the researchers are hopeful given the similarities of cells and mechanisms involved. McKenna says since the technique works in the laboratory, the findings provide “strong preliminary evidence” it could work in living creatures. They are already planning the next phase involving animals and hearing loss.
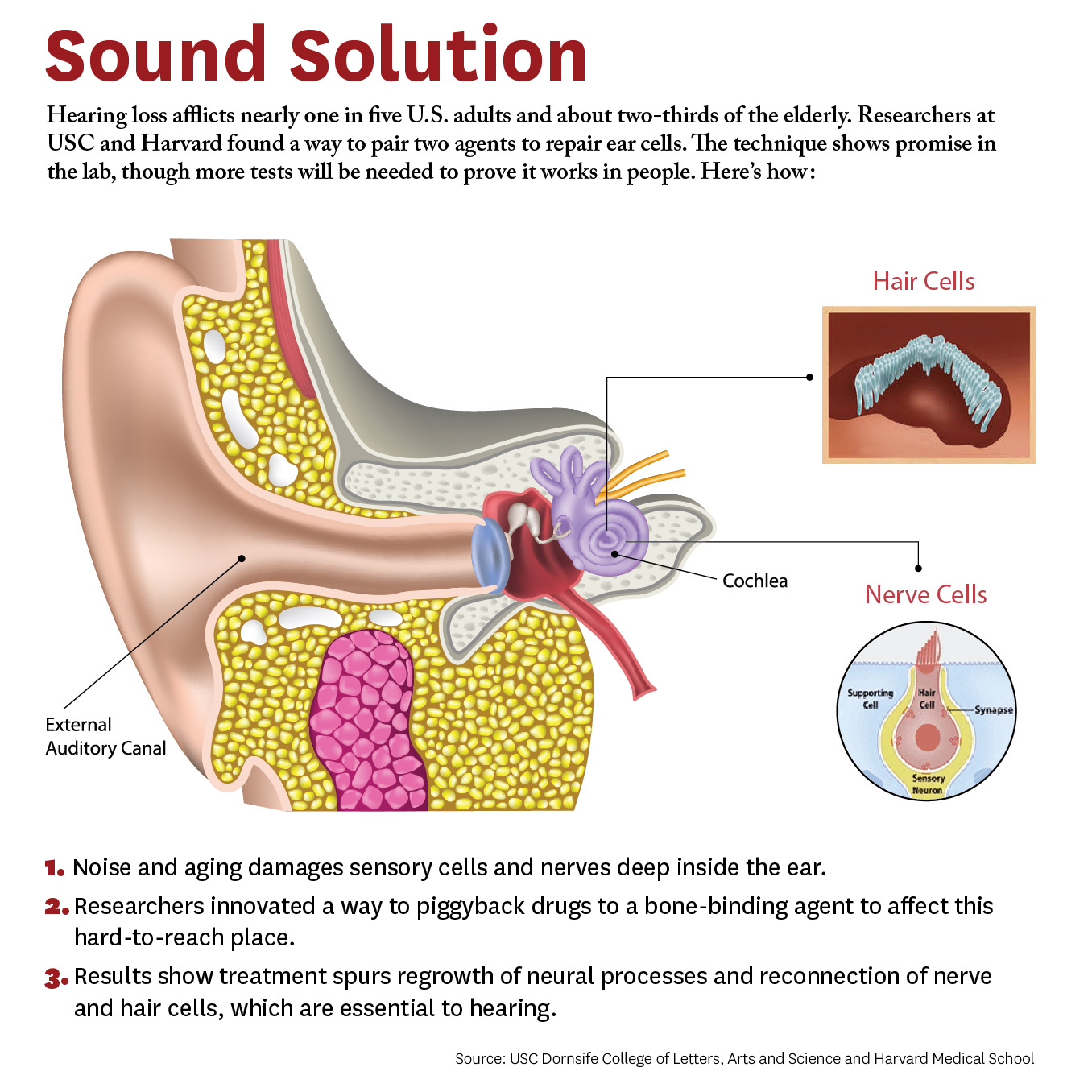
The study breaks new ground because researchers developed a novel drug-delivery method. Specifically, it targets the cochlea, a snail-like structure in the inner ear where sensitive cells convey sound to the brain. Hearing loss occurs due to aging or exposure to noise at unsafe levels. Over time, hair-like sensory cells and bundles of neurons that transmit their vibrations break down, as do ribbon-like synapses, which connect the cells.
The researchers designed a molecule combining 7,8-dihydroxyflavone, which mimics a protein critical for development and function of the nervous system, and bisphosphonate, a type of drug that sticks to bones. This pairing delivered the breakthrough solution, the researchers say, as neurons responded to the molecule and regenerated synapses in mouse ear tissue. This led to the repair of the hair cells and neurons, which are essential to hearing.
“We’re not saying it’s a cure for hearing loss,” McKenna says. “It’s a proof of principle for a new approach that’s extremely promising. It’s an important step that offers a lot of hope.” Hearing loss affects two thirds of people over 70 years and 17 percent of all adults in the United States, and it is expected to nearly double in 40 years.
This is adapted from "Hearing Loss Study at USC, Harvard Shows Hope for Millions" on the USC News website. The authors of the April 4, 2018, Bioconjugate Chemistry study, “Bisphosphonate-Linked TrkB Agonist: Cochlea-Targeted Delivery of a Neurotrophic Agent as a Strategy for the Treatment of Hearing Loss,” include lead researcher Judith S. Kempfle, as well as Christine Hamadani, Nicholas Koen, Albert S. Edge, and David H. Jung of Harvard Medical School and The Eaton-Peabody Laboratories/Massachusetts Eye and Ear in Boston. Kempfle is also affiliated with the University of Tübingen Medical Center. Corresponding author Charles E. McKenna, as well as Kim Nguyen and Boris A. Kashemirov, are at USC Dornsife.
The research was supported by the American Academy of Otolaryngology–Head and Neck Surgery Herbert Silverstein Otology and Neurotology Research Award, an American Otological Society Research Grant, and a grant from the National Institute of Deafness and other Communicative Disorders (R01 DC007174).