With central inhibition lowered, signals that are typically dampened are able to be perceived, potentially resulting in tinnitus. Our paper also showed the utility of measuring central inhibition through cortical auditory evoked potentials (CAEPs), which are electrical responses in the brain that reveal levels of central inhibition.
Stability in an Unstable World
By studying the mouse brain, Balmer and Trussell have now mapped the direct and indirect circuits that carry sensory information to the vestibular cerebellum. Both types of input activate cells within the vestibular cerebellum called unipolar brush cells (UBCs).
Improved TMC1 Gene Therapy Restores Hearing and Balance in Mice
Half of all inner ear disorders, which have a negative impact on hearing and/or balance, are caused by genetic mutations. A study published in January 2019 in Nature Communications demonstrates the effectiveness of a gene therapy targeting one specific gene mutation, TMC1 (transmembrane channel-like 1).
Single-Cell RNA Sequencing Reveals More Clues for Hair Cell Regeneration
To study the genetic program of hair cell regeneration in zebrafish, we sequenced the RNA of individual cells within neuromasts, allowing us to classify cell types based on their gene expression signature.
Hearing Restoration Project Scientific Director to Lead University’s Research Enterprise
Peter Barr-Gillespie, Ph.D., will be Oregon Health & Science University’s (OHSU) first chief research officer and executive vice president, effective Jan. 1, 2019. Barr-Gillespie has served as interim senior vice president for research at OHSU since 2017.
First Study to Examine Cognitive Development in Deaf Babies Finds Differences Begin in Infancy
A noise injury worsens readily. For hyperacusis sufferers such as myself, quiet makes the condition better; noise makes it worse. Among sufferers this is indisputable, but medical practitioners bizarrely treat quiet as harmful.
Shared Knowledge Is Power
ARO provides auditory and vestibular researchers opportunities present their latest findings and engage in meaningful conversations with one another. If one scientist presents an idea to an audience of 100 scientists, she’s just created the possibility for 100 new ideas will form.
New Insights into the Development of the Hair Cell Bundle
By Yishane Lee
Recent genetic studies have identified that the protein Ripor2 (formerly known as Fam65b) is an important molecule for hearing. It localizes to the stereocilia of auditory hair cells and causes deafness when mutations disrupt its function.
In a study published in the Journal of Molecular Medicine in November 2018, Oscar Diaz-Horta, Ph.D., a 2017 Emerging Research Grants (ERG) scientist, and colleagues further show the role the protein plays by demonstrating how it interacts with other proteins during the development of the hair cell bundle. The team found that the absence of Ripor2 changes the orientation of the hair cell bundle, which in turn affects hearing ability.
Ripor2 interacts with Myh9, a protein encoded by a known deafness gene, and Myh9 is expressed in the hair cell bundle stereocilia as well as its kinocilia (apices). The team found that the absence of Ripor2 means that Myh9 is low in abundance. In the study, Ripor2-deficient mice developed hair cell bundles with atypically localized kinocilia and reduced abundance of a phosphorylated form of Myh9. (Phosphorylation is a cellular process critical for protein function.)
Another specific kinociliary protein, acetylated alpha tubulin, helps stabilize cell structures. The researchers found it is also reduced in the absence of Ripor2.
The study concludes that Ripor2 deficiency affects the abundance and/or role of proteins in stereocilia and kinocilia, which negatively affects the structure and function of the auditory hair cell bundle. These newly detailed molecular aspects of hearing will help to better understand how, when these molecular actions are disrupted, hearing loss occurs.
A 2017 ERG scientist funded by the Children’s Hearing Institute (CHI), Oscar Diaz-Horta, Ph.D., was an assistant scientist in the department of human genetics at the University of Miami. He passed away suddenly in August 2018, while this paper was in production. HHF and CHI both send our deepest condolences to Diaz-Horta’s family and colleagues.
We need your help supporting innovative hearing and balance science through our Emerging Research Grants program. Please make a contribution today.
Disrupted Nerve Cell Function and Tinnitus
By Xiping Zhan, Ph.D.
Tinnitus is a condition in which one hears a ringing and/or buzzing sound in the ear without an external sound source, and as a chronic condition it can be associated with depression, anxiety, and stress. Tinnitus has been linked to hearing loss, with the majority of tinnitus cases occurring in the presence of hearing loss. For military service members and individuals who are constantly in an environment where loud noise is generated, it is a major health issue.
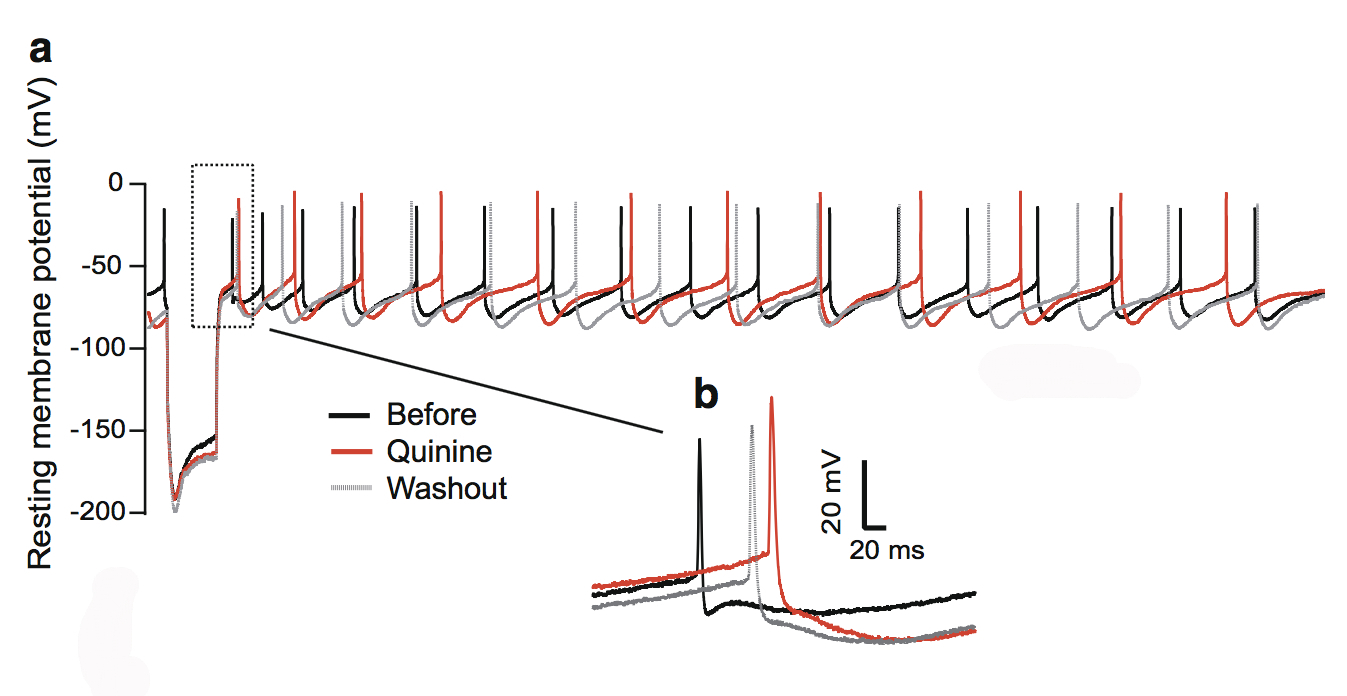
This figure shows the quinine effect on the physiology of dopaminergic neurons in the substantia nigra, a structure in the midbrain.
During this phantom ringing/buzzing sensation, neurons in the auditory cortex continue to fire in the absence of a sound source, or even after deafferentation following the loss of auditory hair cells. The underlying mechanisms of tinnitus are not yet known.
In our paper published in the journal Neurotoxicity Research in July 2018, my team and I examined chemical-induced tinnitus as a side effect of medication. Tinnitus patients who have chemical-induced tinnitus comprise a significant portion of all tinnitus sufferers, and approaching this type of tinnitus can help us to understand tinnitus in general.
We focused on quinine, an antimalarial drug that also causes hearing loss and tinnitus. We theorized this is due to the disruption of dopamine neurons rather than cochlear hair cells through the blockade of neuronal ion channels in the auditory system. We found that dopamine neurons are more sensitive than the hair cells or ganglion neurons in the auditory system. To a lesser extent, quinine also causes muscle reactions such as tremors and spasms (dystonia) and the loss of control over body movements (ataxia).
As dopaminergic neurons (nerve cells that produce the neurotransmitter dopamine) are implicated in playing a role in all of these diseases, we tested the toxicity of quinine on induced dopaminergic neurons derived from human pluripotent stem cells and isolated dopaminergic neurons from the mouse brain.
We found that quinine can affect the basic physiological function of dopamine neurons in humans and mice. Specifically, we found it can target and disturb the hyperpolarization-dependent ion channels in dopamine neurons. This toxicity of quinine may underlie the movement disorders and depression seen in quinine overdoses (cinchonism), and understanding this mechanism will help to learn how dopamine plays a role in tinnitus modulation.
A 2015 ERG scientist, Xiping Zhan, Ph.D., received the Les Paul Foundation Award for Tinnitus Research. He is an assistant professor of physiology and biophysics at Howard University in Washington, D.C. One figure from the paper appeared on the cover of the July 2018 issue of Neurotoxicity Research.
We need your help supporting innovative hearing and balance science through our Emerging Research Grants program. Please make a contribution today.
Headlines in Hearing Restoration
By Yishane Lee
The cornerstone of Hearing Health Foundation for six decades has been funding early-career hearing and balance researchers through its Emerging Research Grants (ERG) program. Many ERG scientists have gone on to obtain prestigious National Institutes of Health (NIH) funding to continue their HHF-funded research; since 1958, each dollar awarded to ERG scientists by HHF has been matched by NIH investments of more than $90. Within the scientific community, ERG is a competitive grant awarded to the most promising investigators, and we’re always especially pleased when our ERG alumni who are now also members of or affiliated with our Hearing Restoration Project consortium make headlines in the mainstream news for their scientific breakthroughs.
Hair cells in the mouse cochlea courtesy of the lab of Hearing Restoration Project (HRP) member Andy Groves, Ph.D., Baylor College of Medicine.
Ronna Hertzano, M.D., Ph.D. (2009–10): Hearing Restoration Project consortium member Hertzano, an associate professor at the University of Maryland School of Medicine, and colleagues identified a gene, Ikzf2, that acts as a key regulator for outer hair cells whose loss is a major cause of age-related hearing loss. The Ikzf2 gene encodes helios, a transcription factor (a protein that controls the expression of other genes). The mutation of the gene in mice impairs the activity of helios in the mice, leading to an outer hair cell deficit.
Reporting in the Nov. 21, 2018, issue of Nature, the team tested whether the opposite effect could be created—if an abundance of helios could boost the population of outer hair cells. They introduced a virus engineered to overexpress helios into the inner ear hair cells of newborn mice, and found that some mature inner hair cells became more like outer hair cells by exhibiting electromotility, a property limited to outer hair cells. The finding that helios can drive inner hair cells to adopt critical outer hair cell characteristics holds promise for future treatments of age-related hearing loss.
Patricia White, Ph.D. (2009, 2011), with Hearing Restoration Project member Albert Edge, Ph.D.: White, a research associate professor at the University of Rochester Medical Center, Edge, a professor of otolaryngology at Massachusetts Eye and Ear and Harvard Medical School, and team have been able to regrow the sensory hair cells found in the mouse cochlea. The study, published in the European Journal of Neuroscience on Sep. 30, 2018, builds on White’s prior research that identified a family of receptors called epidermal growth factor (EGF) that is responsible for activating supporting cells in the auditory organs of birds. When triggered, these cells proliferate and foster the generation of new sensory hair cells. In mice, EGF receptors are expressed but do not drive regeneration of hair cells, so it could be that as mammals evolved, the signaling pathway was altered.
The new study aimed to unblock the regeneration of hair cells and also integrate them with nerve cells, so they are functional, by switching the EGF signaling pathway to act as it does in birds. The team focused on a specific receptor called ERBB2, found in supporting cells. They used a number of methods to activate the EGF signaling pathway: a virus targeting ERBB2 receptors; mice genetically altered to overexpress activated ERBB2; and two drugs developed to stimulate stem cell activity in the eye and pancreas that are already known to activate ERBB2 signaling. The researchers found that activating the ERBB2 pathway triggered a cascading series of cellular events: Supporting cells began to proliferate and started the process of activating other neighboring stem cells to lead to “apparent supernumerary hair cell formation,” and these hair cells’ integration with the network of neurons was also supported.
This was prepared using press materials from the University of Maryland and the University of Rochester. For more, see hhf.org/hrp.