A noise injury worsens readily. For hyperacusis sufferers such as myself, quiet makes the condition better; noise makes it worse. Among sufferers this is indisputable, but medical practitioners bizarrely treat quiet as harmful.
Shared Knowledge Is Power
ARO provides auditory and vestibular researchers opportunities present their latest findings and engage in meaningful conversations with one another. If one scientist presents an idea to an audience of 100 scientists, she’s just created the possibility for 100 new ideas will form.
New Insights into the Development of the Hair Cell Bundle
By Yishane Lee
Recent genetic studies have identified that the protein Ripor2 (formerly known as Fam65b) is an important molecule for hearing. It localizes to the stereocilia of auditory hair cells and causes deafness when mutations disrupt its function.
In a study published in the Journal of Molecular Medicine in November 2018, Oscar Diaz-Horta, Ph.D., a 2017 Emerging Research Grants (ERG) scientist, and colleagues further show the role the protein plays by demonstrating how it interacts with other proteins during the development of the hair cell bundle. The team found that the absence of Ripor2 changes the orientation of the hair cell bundle, which in turn affects hearing ability.
Ripor2 interacts with Myh9, a protein encoded by a known deafness gene, and Myh9 is expressed in the hair cell bundle stereocilia as well as its kinocilia (apices). The team found that the absence of Ripor2 means that Myh9 is low in abundance. In the study, Ripor2-deficient mice developed hair cell bundles with atypically localized kinocilia and reduced abundance of a phosphorylated form of Myh9. (Phosphorylation is a cellular process critical for protein function.)
Another specific kinociliary protein, acetylated alpha tubulin, helps stabilize cell structures. The researchers found it is also reduced in the absence of Ripor2.
The study concludes that Ripor2 deficiency affects the abundance and/or role of proteins in stereocilia and kinocilia, which negatively affects the structure and function of the auditory hair cell bundle. These newly detailed molecular aspects of hearing will help to better understand how, when these molecular actions are disrupted, hearing loss occurs.
A 2017 ERG scientist funded by the Children’s Hearing Institute (CHI), Oscar Diaz-Horta, Ph.D., was an assistant scientist in the department of human genetics at the University of Miami. He passed away suddenly in August 2018, while this paper was in production. HHF and CHI both send our deepest condolences to Diaz-Horta’s family and colleagues.
We need your help supporting innovative hearing and balance science through our Emerging Research Grants program. Please make a contribution today.
Disrupted Nerve Cell Function and Tinnitus
By Xiping Zhan, Ph.D.
Tinnitus is a condition in which one hears a ringing and/or buzzing sound in the ear without an external sound source, and as a chronic condition it can be associated with depression, anxiety, and stress. Tinnitus has been linked to hearing loss, with the majority of tinnitus cases occurring in the presence of hearing loss. For military service members and individuals who are constantly in an environment where loud noise is generated, it is a major health issue.
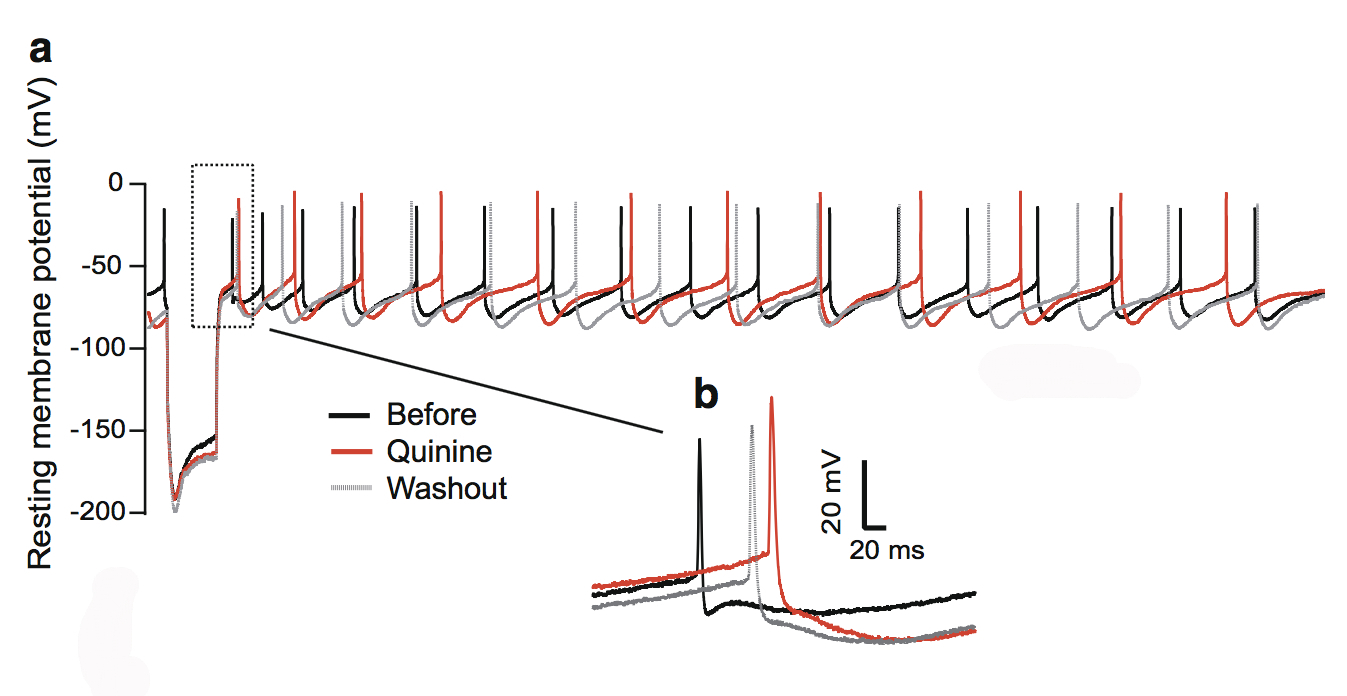
This figure shows the quinine effect on the physiology of dopaminergic neurons in the substantia nigra, a structure in the midbrain.
During this phantom ringing/buzzing sensation, neurons in the auditory cortex continue to fire in the absence of a sound source, or even after deafferentation following the loss of auditory hair cells. The underlying mechanisms of tinnitus are not yet known.
In our paper published in the journal Neurotoxicity Research in July 2018, my team and I examined chemical-induced tinnitus as a side effect of medication. Tinnitus patients who have chemical-induced tinnitus comprise a significant portion of all tinnitus sufferers, and approaching this type of tinnitus can help us to understand tinnitus in general.
We focused on quinine, an antimalarial drug that also causes hearing loss and tinnitus. We theorized this is due to the disruption of dopamine neurons rather than cochlear hair cells through the blockade of neuronal ion channels in the auditory system. We found that dopamine neurons are more sensitive than the hair cells or ganglion neurons in the auditory system. To a lesser extent, quinine also causes muscle reactions such as tremors and spasms (dystonia) and the loss of control over body movements (ataxia).
As dopaminergic neurons (nerve cells that produce the neurotransmitter dopamine) are implicated in playing a role in all of these diseases, we tested the toxicity of quinine on induced dopaminergic neurons derived from human pluripotent stem cells and isolated dopaminergic neurons from the mouse brain.
We found that quinine can affect the basic physiological function of dopamine neurons in humans and mice. Specifically, we found it can target and disturb the hyperpolarization-dependent ion channels in dopamine neurons. This toxicity of quinine may underlie the movement disorders and depression seen in quinine overdoses (cinchonism), and understanding this mechanism will help to learn how dopamine plays a role in tinnitus modulation.
A 2015 ERG scientist, Xiping Zhan, Ph.D., received the Les Paul Foundation Award for Tinnitus Research. He is an assistant professor of physiology and biophysics at Howard University in Washington, D.C. One figure from the paper appeared on the cover of the July 2018 issue of Neurotoxicity Research.
We need your help supporting innovative hearing and balance science through our Emerging Research Grants program. Please make a contribution today.
Headlines in Hearing Restoration
By Yishane Lee
The cornerstone of Hearing Health Foundation for six decades has been funding early-career hearing and balance researchers through its Emerging Research Grants (ERG) program. Many ERG scientists have gone on to obtain prestigious National Institutes of Health (NIH) funding to continue their HHF-funded research; since 1958, each dollar awarded to ERG scientists by HHF has been matched by NIH investments of more than $90. Within the scientific community, ERG is a competitive grant awarded to the most promising investigators, and we’re always especially pleased when our ERG alumni who are now also members of or affiliated with our Hearing Restoration Project consortium make headlines in the mainstream news for their scientific breakthroughs.
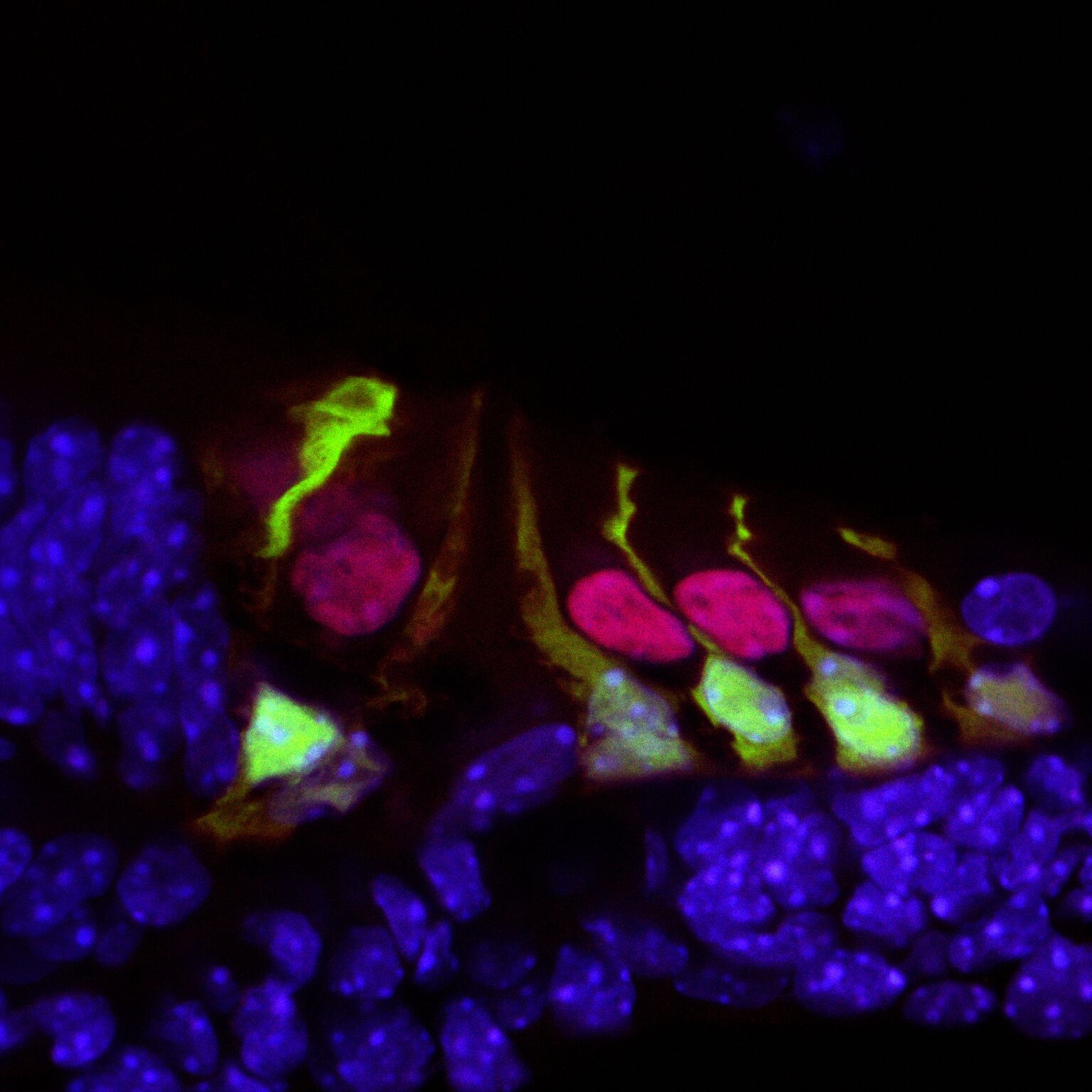
Hair cells in the mouse cochlea courtesy of the lab of Hearing Restoration Project (HRP) member Andy Groves, Ph.D., Baylor College of Medicine.
Ronna Hertzano, M.D., Ph.D. (2009–10): Hearing Restoration Project consortium member Hertzano, an associate professor at the University of Maryland School of Medicine, and colleagues identified a gene, Ikzf2, that acts as a key regulator for outer hair cells whose loss is a major cause of age-related hearing loss. The Ikzf2 gene encodes helios, a transcription factor (a protein that controls the expression of other genes). The mutation of the gene in mice impairs the activity of helios in the mice, leading to an outer hair cell deficit.
Reporting in the Nov. 21, 2018, issue of Nature, the team tested whether the opposite effect could be created—if an abundance of helios could boost the population of outer hair cells. They introduced a virus engineered to overexpress helios into the inner ear hair cells of newborn mice, and found that some mature inner hair cells became more like outer hair cells by exhibiting electromotility, a property limited to outer hair cells. The finding that helios can drive inner hair cells to adopt critical outer hair cell characteristics holds promise for future treatments of age-related hearing loss.
Patricia White, Ph.D. (2009, 2011), with Hearing Restoration Project member Albert Edge, Ph.D.: White, a research associate professor at the University of Rochester Medical Center, Edge, a professor of otolaryngology at Massachusetts Eye and Ear and Harvard Medical School, and team have been able to regrow the sensory hair cells found in the mouse cochlea. The study, published in the European Journal of Neuroscience on Sep. 30, 2018, builds on White’s prior research that identified a family of receptors called epidermal growth factor (EGF) that is responsible for activating supporting cells in the auditory organs of birds. When triggered, these cells proliferate and foster the generation of new sensory hair cells. In mice, EGF receptors are expressed but do not drive regeneration of hair cells, so it could be that as mammals evolved, the signaling pathway was altered.
The new study aimed to unblock the regeneration of hair cells and also integrate them with nerve cells, so they are functional, by switching the EGF signaling pathway to act as it does in birds. The team focused on a specific receptor called ERBB2, found in supporting cells. They used a number of methods to activate the EGF signaling pathway: a virus targeting ERBB2 receptors; mice genetically altered to overexpress activated ERBB2; and two drugs developed to stimulate stem cell activity in the eye and pancreas that are already known to activate ERBB2 signaling. The researchers found that activating the ERBB2 pathway triggered a cascading series of cellular events: Supporting cells began to proliferate and started the process of activating other neighboring stem cells to lead to “apparent supernumerary hair cell formation,” and these hair cells’ integration with the network of neurons was also supported.
This was prepared using press materials from the University of Maryland and the University of Rochester. For more, see hhf.org/hrp.
Recording Electrical Responses to Improve the Diagnosis of Hearing Conditions
By Yishane Lee
Electrocochleography (ECochG) is a method to record electrical responses from the inner ear and the auditory nerve in the first 5 milliseconds after a sound stimulus, such as a click or tone burst. These stimuli can be adjusted for repetition rate and polarity, and recordings can also be taken from either the ear canal, eardrum, or through the eardrum. The main components of ECochG response are the summating potential (SP), from the sensory hair cells in the cochlea, and the action potential (AP) of auditory nerve fibers. In an October 2018 paper in the journal Canadian Audiologist, 2015 ERG scientist Wafaa Kaf, Ph.D., reviews the diagnostic applications for using ECochG for Ménière’s disease and cochlear synaptopathy, two conditions that can be difficult to pinpoint, especially early in the disease, and suggests how to improve the use of ECochG as a clinical tool.
Shutterstock
Endolymphatic hydrops, or abnormal fluctuations in inner ear fluid, is believed to be the underlying cause of Ménière’s disease and its associated hearing and balance disorder. ECochG collects information about the SP/AP amplitude and area ratios that can be used to confirm a Ménière’s diagnosis, without relying solely on clinical symptoms.
Since the SP/AP amplitude ratio can vary among known Ménière’s patients, Kaf suggests including data about the SP/AP area ratio as well can help with diagnosing the disease. To further distinguish Ménière’s, Kaf suggests using ECochG AP latencies, and, building on her prior research, the effect of fast click rates on the auditory nerve latency and amplitude. Using the continuous loop averaging deconvolution technique, various properties of the SP and AP waveforms are easier to identify and parse. Results suggest that the functions of the cochlear nerve and/or cochlear synapses are damaged in Ménière’s. Earlier research that shows an abnormal acoustic reflex decay in about a quarter of Ménière’s patients, and a reduced number of synapses between inner hair cells and auditory nerve fibers, underscores the presence of nerve damage in Ménière’s.
Cochlear synaptopathy is a noise-induced or age-related dysfunction that is also causing reduced synapses between inner ear hair cells and auditory nerve fibers, resulting in tinnitus, hyperacusis, and difficulty hearing in noise despite normal hearing sensitivity. ECochG may help with its diagnosis, especially given that traditional audiograms and hearing tests have been found to miss this “hidden hearing loss.” The use of both the SP/AP amplitude and area ratios and specific auditory brainstem responses can help confirm this condition and distinguish it from Ménière’s disease.
ECochG can also be used to help confirm the diagnosis of auditory neuropathy spectrum disorder, a problem with the way sound is transmitted between the inner ear and the brain, and other inner ear disorders. The technique can also be used to monitor ear responses, real-time, during surgeries such as a stapedectomy, endolymphatic shunt, and cochlear implantation, additional instances demonstrating how ECochG holds promise for expanded use in the clinic.
A 2015 ERG scientist funded by The Estate of Howard F. Schum, Wafaa Kaf, Ph.D., is a professor of audiology at Missouri State University.
We need your help supporting innovative hearing and balance science through our Emerging Research Grants program. Please make a contribution today.
The People Behind the Science
By Yishane Lee
Eight years ago we introduced a column called “Meet the Researcher.” Placed on the last page of the magazine (prime editorial real estate!), the MTR column was designed as a way to give our Emerging Research Grants (ERG) scientists a place to talk about their ERG project in more detail—and in lay terms for our readers—including its genesis, planned execution, and future goals.
Credit: Jane G. Photography
“Meet the Researcher” also is an opportunity for us to glimpse the person behind the science, with the researchers sharing how they became interested in their field and whether they have any personal connection to hearing conditions. Perhaps not surprisingly, many researchers do become interested in hearing and balance science as a result of their own experience with hearing loss. For instance, 2010 ERG scientist Judith Kempfle, M.D., told us she received an artificial eardrum at age 13, after many ear infections that her brother also got when they were kids growing up in Germany. With her ERG funded by the Royal Arch Masons General Grand Chapter International, Kempfle has gone on to work on many papers with Hearing Restoration Project member Albert Edge, Ph.D. (including a recent one about the effort to deliver drugs directly to the inner ear).
Ed Bartlett, Ph.D., Purdue University
Also a Royal Arch Masons grantee, 2011 ERG scientist Ed Bartlett, Ph.D., who published research on the lasting effects of blast shock waves on auditory processing, remembers asking his teacher whether we actually hear thoughts or if it something else. “So, I guess I was destined for auditory neuroscience,” says Bartlett, who also earned ERG funding in 2003, 2004, and 2009.
2011 and 2012 ERG scientist Regie Santos-Cortez, M.D., Ph.D., who earned the Collette Ramsey Baker Award named after HHF’s founder, spoke about the challenges of getting access to genetic information for her study that eventually pinpointed a gene mutation linked to a predisposition for ear infections. 2012 ERG scientist Bradley J. Walters, Ph.D., says he started out studying evolutionary biology, switched to studying regenerating damaged brain tissue, and then switched to hearing research. “I realized a lot of the ideas I had been working on in the brain could be applied to the ear,” he says. A 2017 paper he coauthored described successfully using gene therapy to regenerate hair cells in adult mice.
Alan Kan, Ph.D., University of Wisconsin, Madison
An early love of logic puzzles for 2013 ERG scientist Alan Kan, Ph.D., a Royal Arch Masons grantee, turned into studying audio engineering and his 2018 paper looking at how to improve speech understanding among people who use bilateral cochlear implants. Fellow 2013 Royal Arch Masons recipient Ross Maddox, Ph.D., remembers varying how he cupped his hands over his ears to get different sounds, leading to an interest in auditory processes and, eventually, research on how auditory and visual input is synthesized to understand sound.
After 26 years as a clinical audiologist, Royal Arch Masons 2014 ERG scientist Samira Anderson, Ph.D., switched to research. “Part of my motivation came from working with patients who struggled with their hearing aids,” she says. “I was frustrated that I was unable to predict who would benefit from hearing aids based on the results of audiological evaluations.” She produced three papers on the topic, bringing us closer to improving fit for and increasing the use of hearing aids.
Likewise, fellow Royal Arch Masons grantee Srikanta Mishra, Ph.D., produced two papers, one in 2017 and one in 2018, on children’s hearing that stemmed from his 2014 ERG grant—work that also led to a prestigious National Institutes on Deafness and Other Communication Disorders grant. And we liked the backstory for 2014 ERG scientist Brad Buran, Ph.D., so much that we put him on the cover of our magazine. Buran, who wears cochlear implants, multitasks during happy hour with his colleagues. “In an environment where it’s hard to hear,” he says, “within an hour they have all the information they need to use Cued Speech,” which uses visual representations of phonemes.
Beula Magimairaj, Ph.D., University of Central Arkansas
In 2015, we expanded our coverage of ERG recipients, so that every grantee is profiled in a “Meet the Researcher” column, all available online. Three papers resulted from the Royal Arch Masons grant received by 2015 ERG scientist Beula Magimairaj, Ph.D., and her research into children’s speech perception in noise and auditory processing (the third paper is in press). Funded by Hyperacusis Research Ltd., 2015 ERG scientist Kelly Radziwon, Ph.D., has managed to create a reliable animal model for loudness hyperacusis (essentially, inducing loudness intolerance in a rat and making sure it reacts to gradually increasing sound intensities) as well as finding a potential link between neuroinflammation and hyperacusis. 2015 and 2016 ERG scientist Wafaa Kaf, Ph.D.—who has 18 other family members (and counting!) who work in science—has been investigating Ménière’s disease, publishing on improving its diagnosis as it can be mistaken for other conditions, and the use of electrocochleography (ECochG) for diagnosing and monitoring the hearing and balance disorder.
Elizabeth McCullagh, Ph.D., University of Colorado
A karaoke fan who admits he “cannot resist Celine Dion,” Royal Arch Masons 2016 ERG scientist Richard Felix II, Ph.D. published on the greater-than-expected role of lower-level brain regions on speech processing. Fellow Royal Arch Masons grantee, 2016 ERG scientist Elizabeth McCullagh, Ph.D., makes her own cheese and beer in between uncovering new clues to sound localization problems in the genetic condition known as Fragile X syndrome, which can lead to autism.
Rahul Mittal, Ph.D., University of Miami Miller School of Medicine
2016 ERG scientist Harrison Lin, Ph.D., funded by The Barbara Epstein Foundation Inc., credits his older brother, also an otolaryngologist, for developing in him a love for science. He coauthored a January 2018 JAMA Otolaryngology–Head & Neck Surgery paper that detailed the gap between hearing loss diagnoses and treatments. 2016 ERG scientist Rahul Mital, Ph.D., who says he’d write fiction if not doing research, published an overview of hair cell regeneration, and Julia Campbell, Au.D., Ph.D., whose 2016 grant was funded by the Les Paul Foundation, understands firsthand what is feels like to have tinnitus, a topic she recently published a paper that investigated mild tinnitus in young patients with typical hearing. Hyperacusis Research-funded 2016 ERG scientist Xiying Guan, Ph.D., whose parents grew up doing manual labor in China, published a paper evaluating a treatment for conductive hyperacusis.
Some of our 2017 ERG scientists are already publishing. Royal Arch Masons grantee Inyong Choi, Ph.D., produced research on hybrid cochlear implants, which make use of residual hearing to produce more natural hearing. Oscar Diaz-Horta, Ph.D., whose 2017 ERG grant was funded by the Children’s Hearing Institute, investigated hair cell bundle structure and orientation. Very regretfully, Diaz-Horta died unexpectedly just as this paper was published.
Ian Swinburne, Ph.D., Harvard Medical School
Ian Swinburne, Ph.D., one of our Ménière’s Disease Grants scientists during its inaugural year in 2017, published a paper detailing one possible cause of Ménière’s disease. Swinburne and team discovered a structure in the inner ear’s endolymphatic sac acts a pressure-sensitive relief valve. Its failure may account for problems with inner ear fluid pressure and volume that may lead to hearing and balance disorders, including Ménière’s. “One activity I loved as a child was waterworks: building canals and aqueducts out of sand or dirt and then pouring water through them just to watch it flow,” he says. “Now I recognize an echo of that play in my study of water pressure and flow within the ear.”
We very much look forward to published research from all of our ERG scientists, including our latest crop of 2018 ERG scientists, whose ranks include a former college mascot, a violinist, a horse rider (of a horse named Gandalf), a Tibetan neuroscientist (and cookbook writer), a cricket player, a nonprofit cook who has prepared meals for 50,000 people, a dancer (including in flash mobs), and a builder of airplane scale models. Our ERG scientists deliver surprises of all sorts, from their backgrounds and how they got to where they are to the ground-breaking science they are spearheading in the lab.
We need your help supporting innovative hearing and balance science through our Emerging Research Grants program. Please make a contribution today.
On a Data-Driven Mission
By Peter G. Barr-Gillespie, Ph.D.
The annual meeting of Hearing Health Foundation’s (HHF) Hearing Restoration Project was held in Seattle November 11-12, 2018. We used this meeting to update one another on recent progress on HHF-funded projects, discuss in detail the implications of new data, evaluate the directions of ongoing projects, and plan for the next funding period.
As you may recall, in November 2016 the Hearing Restoration Project (HRP) made a deliberate turn toward funding only the highest-impact science that our group leads the world in researching—we have termed this the “Seattle Plan.” We therefore devoted a substantial portion of our efforts to cross-species comparisons that contrast molecular responses to inner ear sensory hair cell damage in species that regenerate their hair cells, especially chickens and fish, with responses in mice, which like other mammals do not regenerate their hair cells. We also have been examining the “epigenetic” structure of key genes in the mouse, as one hypothesis is that epigenetic modifications of the DNA—that is, the inactivation of genes through chemical changes to the DNA—causes mouse (and human) cells of the cochlea to no longer respond to hair cell damage by regenerating hair cells.
Avian and mammal supporting cell subtypes differ, but Stefan Heller, Ph.D., and team are investigating if an evolutionary homogenous equivalent exists in the organ of Corti, and if this knowledge could be used for hair cell regeneration. Credit: Chris Gralapp / Otolaryngology Head and Neck Surgery (OHNS) - Stanford University School of Medicine
I am happy to report that progress over the past two years on these two major projects has been outstanding. For the cross-species comparisons, Stefan Heller, Ph.D., and Tatjana Piotrowski, Ph.D., reported on single cell analysis of, respectively, chick and fish hair cell organs responding to damage. Using single cell analysis—isolating hundreds to thousands of individual cells and quantifying all of the protein-assembly messages they express—we can determine the molecular pathways by which hair cells are formed during development and regeneration. This approach has always been promising, but this year we have begun to reap the expected benefits, as those projects have given us an unprecedented view of hair cell formation.
The epigenetics project overseen by Neil Segil, Ph.D., has now reached maturity, and using the voluminous data acquired over the past several years his lab has shown how supporting cells (from which we intend to regenerate hair cells) change the epigenetic modification of their DNA so they no longer are able to switch on key genes used for turning them into hair cells. A topic of great interest at the meeting was that of genetic reprogramming: Can we use genes (like transcription factors, proteins that control the transfer of genetic information) or small molecules (which often can be taken orally and still reach their targets) to overcome the epigenetic modification and push supporting cells to turn into hair cells? Preliminary results from Segil’s lab and from others in the field make us optimistic that the reprogramming approach will eventually be part of a regeneration strategy.
We also heard from Seth Ament, Ph.D., a bioinformatics expert we recently recruited to the HRP to explicitly compare our various datasets and find the common threads between them. Ament has used gene expression data from the chick, fish, and mouse, as well as the epigenetic data from the mouse, to hypothesize which genes may be important for hair cell regeneration. As a systems biology specialist, Ament brings a fresh eye to the field of auditory science and has not only identified some of the genes we expected to be important, but new ones as well. His success nicely justifies our cross-species approach, and the bioinformatics comparisons that he has been able to achieve in his initial HRP project have been impressive.
Finally, two other Seattle Plan projects have gone well, including our data-sharing platform called the gEAR (gene Expression Analysis Resource), developed by Ronna Hertzano, M.D., Ph.D., which allows us to analyze our data privately but also to efficiently share data with the public. In addition, John Brigande, Ph.D., reported on his project developing mouse models for testing interesting new genes; his group will be adding several powerful models in the year to come.
The excitement at the meeting extended to our future plans. We agreed that the Seattle Plan was the still the proper course, and we eagerly anticipate more data and results to come from our consortium of researchers. We are truly getting a clearer picture of hair cell regeneration due to the HRP’s efforts. That said, there is a long way to go; our efforts show us how surprisingly intricate biology is, despite knowing from the start that systems like the inner ear are remarkably complex. Nature always has surprises for us, by turns dashing treasured hypotheses while revealing unexpected mechanisms. The HRP is most definitely on track for success, and all of us in the HRP sincerely thank you for your continued support.
HRP scientific director Peter G. Barr-Gillespie, Ph.D., is a professor of otolaryngology at the Oregon Hearing Research Center, a senior scientist at the Vollum Institute, and the interim senior vice president for research, all at Oregon Health & Science University. For more, see hhf.org/hrp.
Empower our life-changing research with a contribution today.
Detailing the Relationships Between Auditory Processing and Cognitive-Linguistic Abilities in Children
According to our framework, cognitive and linguistic factors are included along with auditory factors as potential sources of deficits that may contribute individually or in combination to cause listening difficulties in children.
Measuring Brain Signals Leads to Insights Into Mild Tinnitus
By Julia Campbell, Au.D., Ph.D.
Tinnitus, or the perception of sound where none is present, has been estimated to affect approximately 15 percent of adults. Unfortunately, there is no cure for tinnitus, nor is there an objective measure of the disorder, with professionals relying instead upon patient report.
There are several theories as to why tinnitus occurs, with one of the more prevalent hypotheses involving what is termed decreased inhibition. Neural inhibition is a normal function throughout the nervous system, and works in tandem with excitatory neural signals for accomplishing tasks ranging from motor output to the processing of sensory input. In sensory processing, such as hearing, both inhibitory and excitatory neural signals depend on external input.
For example, if an auditory signal cannot be relayed through the central auditory pathways due to cochlear damage resulting in hearing loss, both central excitation and inhibition may be reduced. This reduction in auditory-related inhibitory function may result in several changes in the central nervous system, including increased spontaneous neural firing, neural synchrony, and reorganization of cortical regions in the brain. Such changes, or plasticity, could possibly result in the perception of tinnitus, allowing signals that are normally suppressed to be perceived by the affected individual. Indeed, tinnitus has been reported in an estimated 30 percent of those with clinical hearing loss over the frequency range of 0.25 to 8 kilohertz (kHz), suggesting that cochlear damage and tinnitus may be interconnected.
However, many individuals with clinically normal hearing report tinnitus. Therefore, it is possible that in this specific population, inhibitory dysfunction may not underlie these phantom perceptions, or may arise from a different trigger other than hearing loss.
One measure of central inhibition is sensory gating. Sensory gating involves filtering out signals that are repetitive and therefore unimportant for conscious perception. This automatic process can be measured through electrical responses in the brain, termed cortical auditory evoked potentials (CAEPs). CAEPs are recorded via electroencephalography (EEG) using noninvasive sensors to record electrical activity from the brain at the level of the scalp.
Cortical auditory evoked potentials (CAEPs) are recorded via electroencephalography (EEG) using noninvasive sensors to record electrical activity from the brain.
In healthy gating function, it is expected that the CAEP response to an initial auditory signal will be larger in amplitude when compared with a secondary CAEP response elicited by the same auditory signal. This illustrates the inhibition of repetitive information by the central nervous system. If inhibitory processes are dysfunctional, CAEP responses are similar in amplitude, reflecting decreased inhibition and the reduced filtering of incoming auditory information.
Due to the hypothesis that atypical inhibition may play a role in tinnitus, we conducted a study to evaluate inhibitory function in adults with normal hearing, with and without mild tinnitus, using sensory gating measures. To our knowledge, sensory gating had not been used to investigate central inhibition in individuals with tinnitus. We also evaluated extended high-frequency auditory sensitivity in participants at 10, 12.5, and 16 kHz—which are frequencies not included in the usual clinical evaluation—to determine if participants with mild tinnitus showed hearing loss in these regions.
Tinnitus severity was measured subjectively using the Tinnitus Handicap Index. This score was correlated with measures of gating function to determine if tinnitus severity may be worse with decreased inhibition.
Our results, published in Audiology Research on Oct. 2, 2018, showed that gating function was impaired in adults with typical hearing and mild tinnitus, and that decreased gating was significantly correlated with tinnitus severity. In addition, those with tinnitus did not show significantly different extended high-frequency thresholds in comparison to the participants without tinnitus, but it was found that better hearing in this frequency range related to worse tinnitus severity.
This result conflicts with the theory that hearing loss may trigger tinnitus, at least in adults with typical hearing, and may indicate that these individuals possess heightened auditory awareness, although this hypothesis should be directly tested.
Overall, it appears that central inhibition is atypical in adults with typical hearing and tinnitus, and that this is not related to hearing loss as measured in clinically or non-clinically tested frequency regions. The cause of decreased inhibition in this population remains unknown, but genetic factors may play a role. We are currently investigating the use of sensory gating as an objective clinical measure of tinnitus, particularly in adults with hearing loss, as well as the networks in the brain that may underlie dysfunctional gating processes.
2016 Emerging Research Grants scientist Julia Campbell, Au.D., Ph.D., CCC-A, F-AAA, received the Les Paul Foundation Award for Tinnitus Research. She is an assistant professor in communication sciences and disorders in the Central Sensory Processes Laboratory at the University of Texas at Austin.
You can empower work toward better treatments and cures for hearing loss and tinnitus. If you are able, please make a contribution today.