Recent research published in JARO by Emerging Research Grants (ERG) recipient Michelle Hastings, Ph.D., and colleagues shows that early administration of a genetic targeting treatment is critically important for repairing outer hair cells and thus rescuing hearing in those with genetic disorders like Usher syndrome.
New Method Enables Systematic Study of Hair Cell Loss and Regeneration in Chickens
By Carol Stoll
Most forms of hearing loss are permanent because damage to inner ear sensory hair cells is irreversible in mammals, including humans. Mammalian vestibular hair cells have the potential to regenerate albeit at a low rate, but the hair cells of the adult mammalian cochlea are not regenerated. Birds, however, have a robust regenerative response to hair cell damage and are able to restore structure and function in inner ear organs. Consequently, the study of the molecular mechanisms that trigger the onset of avian hair cell regeneration in the balance organs as well as in the cochlea is important and may lead to therapies for hearing loss in humans.
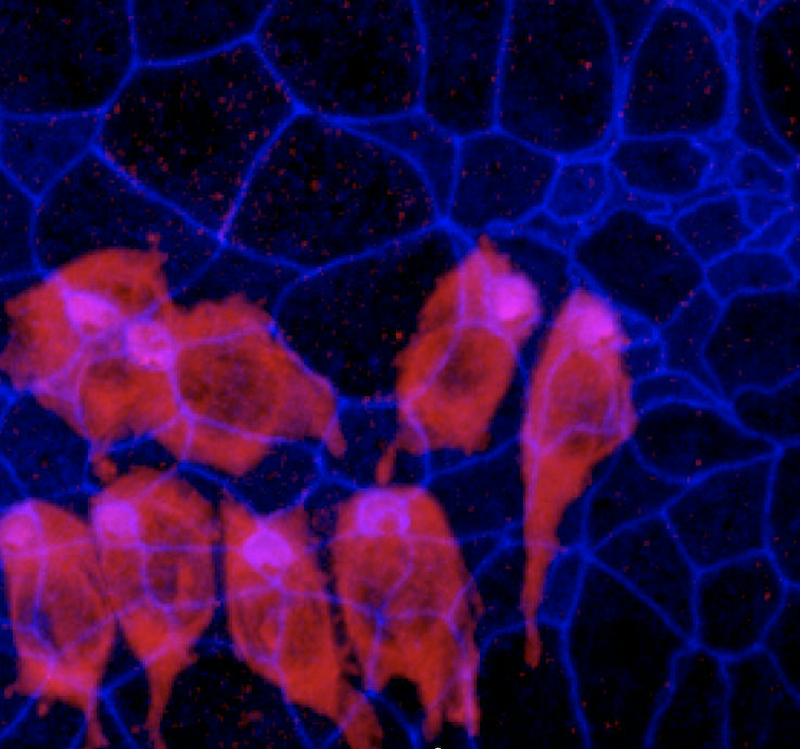
This image shows the undamaged and damaged utricle, an inner ear balance organ, in a chicken. HRP researchers have devised a new method to study the precise timing of hair cell regeneration in chickens using a single surgical application of an ototoxic drug. Photo by Amanda Janesick, Ph.D.
Past experiments that investigate these regeneration mechanisms in living chickens required multiple injections of a drug to induce hair cell loss, making it difficult to determine the exact timing of the regeneration response. A collaboration of two Hearing Restoration Project researchers, Stefan Heller, Ph.D. and Jennifer Stone, Ph.D., and two talented postdoctoral fellows from their laboratories was recently published in Journal of the Association for Research in Otolaryngology identifying a potential solution to this problem. They developed an experimental framework that uses a single ototoxic drug application, enabling them to study the precise onset and timing of hair cell regeneration in vivo.
Heller, Stone, and colleagues performed their experiments on a total of 75 chickens. At seven days of age, the chickens were anesthetized and underwent surgery to eliminate hair cells in the inner ear organs. During the surgery, streptomycin (an ototoxic antibiotic) was delivered to the chicken’s inner ear. At various time points after the surgery, two sensory organs—the utricle, a vestibular organ; and the basilar papilla, the hearing organ—were dissected, labeled for various cellular markers, and analyzed under a microscope. Hair cells and their surrounding supporting cells were counted and observed for damage. EdU, a marker of cell division, was administered to the chickens to determine whether or not new hair cells were generated by cell division. These techniques enabled the researchers to quantitatively characterize the regenerative response of the utricle after damage.
The results of the study demonstrate that surgical application of a single streptomycin dose is a feasible approach to elicit hair cell loss and regeneration in the chicken utricle and basilar papilla. Just hours after streptomycin delivery, hair cell numbers significantly declined and DNA replication was activated. The team was then able to record specific events of the regeneration process, which get initiated around 12 hours after streptomycin-induced hair cell loss, and continue over the course of several days.
Supporting cells produce new hair cells either by converting into a hair cell (direct transdifferentiation), or by dividing, usually asymmetrically, into a supporting cell and a hair cell. Throughout this regenerative response, supporting cell numbers and density in the utricle remain relatively constant, suggesting that there is a mechanism that responds to specific levels of damage and coordinates the individual events of the regeneration process.
The study establishes a framework for the refined study of the two modes of hair cell regeneration in the chicken utricle. The next steps of the work will focus on understanding the exact timing and mechanism of coordination of the regeneration response. With only a single application of streptomycin necessary to induce near-complete hair cell loss in hearing and balance organs, the new animal model allows for study of the entire process including initiation, realization, and termination. The fundamental understanding of the avian regenerative mechanisms may lead to future development of therapies for loss of hearing and balance in humans.
Empower the Hearing Restoration Project's life-changing research. If you are able, please make a contribution today.
2018: Hear's to You
By Nadine Dehgan
From every one of us at Hearing Health Foundation (HHF)—scientists, staff, and volunteers—thank you for your support in 2017 and best wishes for 2018.
Because of folks like you, 2017 was an incredible year in which HHF:
Increased funding for Hearing Restoration Project & Emerging Research Grants by 35%
Launched Ménière's Disease Grants program to better understand & treat this condition
Began to fund critical Ototoxic Drug Research so cancer survivors won't have to live with hearing loss as a result of their life-saving treatments
Advocated for universal newborn hearing screenings, resulting in the passage of the Early Hearing Detection and Intervention Act
Endorsed the Over-the-Counter Hearing Aid Act, which will provide a more affordable and accessible treatment option for adults with mild to moderate hearing loss
Created Faces of Hearing Loss to show that hearing and balance disorders affect all of us
Received Top Ratings from all Charity Watchdogs including Consumer Reports and BBB
With hearts full of gratitude, we look forward to the work to be done in 2018—HHF’s 60th anniversary year.
With your help, HHF will continue to fund groundbreaking discoveries for the tens of millions of Americans with hearing loss and tinnitus—among whom is Ethan, 6, born with bilateral (in both ears) hearing loss and fortunate to receive early intervention.
Ethan is a first-grader who loves his sisters, soccer, reading, math, and martial arts. Until a cure for hearing loss is realized, he will be dependent on hearing aids or other treatments.
New scientific findings in 2018—empowered by you—can change the future of hearing loss for Ethan and so many others. I look forward to updating you on progress made.
New Grantees Will Advance Understanding of Ménière's Disease
By Lauren McGrath
Hearing Health Foundation's (HHF) newly established Ménière's Disease Grants (MDG) program will significantly advance our understanding of the mechanisms of Ménière's Disease. In 2018, two innovators will have the opportunity to investigate the disorder's diagnosis and treatment.
Ménière's Disease is a disorder of the inner ear that causes episodes of vertigo as a result of fluid that fills the tubes of the inner ear. In addition to dizziness and nausea, Ménière's attacks can cause some loss of hearing in one or both ears and a constant ringing sound. The causes of Ménière's remain unknown and a cure has yet to be identified. The National Institutes of Health estimates that 615,000 individuals in the United States live with the disorder.
Two grants have recently been awarded for 2018 for innovative Ménière's Disease research. Both grantees were also previously funded by HHF’s Emerging Research Grants (ERG) program.
Gail Ishiyama, M.D.
Gail Ishiyama, M.D. of UCLA David Geffen School of Medicine is focusing on cellular and molecular biology of the microvasculature in the macula utricle of patients diagnosed with Ménière’s disease. Her project will provide greater information on the blood labyrinthine barrier and allow for the development of interventions that prevent the progression of hearing loss and stop the disabling vertigo in Ménière’s disease patients.
Ian Swinburne, Ph.D.
Ian Swinburne, Ph.D. of Harvard Medical School is classifying the endolymphatic duct and sac's cell types and their gene sets using high-throughput single-cell transcriptomics. His work will generate a list of endolymphatic sac cell types and the genes governing their function, which will aid in Ménière's diagnosis (which can be delayed due to the range of fluctuating symptoms) and the development of a targeted drug or gene therapy.
HHF is grateful for the opportunity to fund Drs. Ishiyama and Swinburne. If you are interested in naming a research grant in any discipline within the hearing and balance space, please contact development@hhf.org.
The Gap Between Self-Reported Hearing Loss and Treatment Patterns
By Carol Stoll
Hearing loss is one of the most prevalent chronic conditions in the U.S. and has been associated with negative physical, social, cognitive, economic, and emotional consequences. Despite the high prevalence of hearing loss, substantial gaps in the utilization of amplification options, including hearing aids and cochlear implants (CI), have been identified. Harrison Lin, M.D., a 2016 Emerging Research Grants recipient, along with colleagues, recently published a paper in JAMA Otolaryngology–Head & Neck Surgery that investigates the contemporary prevalence, characteristics, and patterns of specialty referral, evaluation, and treatment of hearing difficulty among adults in the U.S.
Unlike this man who is having his hearing tested, a large number of individuals in the U.S. who experience hearing difficulties are not seeking treatment. Photo source: Bundesinnung Hörakustiker, Flickr.
The researchers did a cross-sectional analysis of responses from a nationwide representative sample of adults who participated in the 2014 National Health Interview Survey and responded to hearing health questions. The data collected included demographic information as well as self-reported hearing status, functional hearing, laterality (hearing ability in each ear), onset, and primary cause (if known) of the hearing loss. In addition, the team analyzed specific data regarding hearing-related clinician visits, hearing tests, referrals to hearing specialist, and utilization of hearing aids and CIs.
Overall, 36,690 records were included in the analysis, which extrapolated to an estimated 239.6 million adults in the U.S. Nearly 17 percent indicated their hearing was less than “excellent/good,” ranging from “a little trouble hearing” to “deaf.” Approximately 21 percent of respondents had visited a physician for hearing problems in the preceding five years. Of these, 33 percent were referred to an otolaryngologist and 27 percent were referred to an audiologist. Of the adults who indicated their hearing from “a little trouble hearing” to being “deaf,” 32 percent had never seen a clinician for hearing problems and 28 percent had never had their hearing tested.
The study shows that there are considerable gaps between self-reported hearing loss and patients receiving medical evaluation and recommended treatments for hearing loss. Increased awareness among clinicians regarding the burden of hearing loss, the importance of early detection and medically evaluating hearing loss, and available amplification and CI options can contribute to improved care for individuals with hearing difficulty. Future studies are warranted to further investigate the observed trends of this study.
Harrison W. Lin, M.D., is a 2016 Emerging Research Grants recipient. His grant was generously funded by funded by The Barbara Epstein Foundation, Inc.
We need your help supporting innovative hearing and balance science through our Emerging Research Grants program. Please make a contribution today.
Cellular Changes and Ménière’s Disease Symptoms
By Carol Stoll
Ménière’s disease is characterized by fluctuating hearing loss, vertigo, tinnitus, and ear fullness, but the causes of these symptoms are not well understood. Past research has suggested that a damaged blood labyrinthine barrier (BLB) in the inner ear may be involved in the pathophysiology of inner ear disorders. Hearing Health Foundation (HHF)’s 2016 Emerging Research Grants (ERG) recipient Gail Ishiyama, M.D., was the first to test this proposition by using electron microscopy to analyze the BLB in both typical and Ménière’s disease patients. Ishiyama’s research was fully funded by HHF and was recently published in Nature publishing group, Scientific Reports.
The BLB in a Meniere’s disease capillary. a) Capillary located in the stroma of the macula utricle from a Meniere’s subject (55-year-old-male). The lumen (lu) of the capillary is narrow, vascular endothelial cells (vec) are swollen and the cytoplasm is vacuolated (pink asterisks). b. Diagram showing the alterations in the swollen vec, microvacuoles are also abundant (v). Abbreviations, rbc: red blood cells, tj: tight junctions, m: mitochondria, n: cell nucleus, pp: pericyte process; pvbm: perivascular basement membrane. Bar is 2 microns.
The BLB is composed of a network of vascular endothelial cells (VECs) that line all capillaries in the inner ear organs to separate the vasculature (blood vessels) from the inner ear fluids. A critical function of the BLB is to maintain proper composition and levels of inner ear fluid via selective permeability. However, the inner ear fluid space in patients with Ménière’s has been shown to be ballooned out due to excess fluid. Additionally, the group had identified permeability changes in magnetic resonance imaging studies of Meniere’s patients, which may be an indication of BLB malfunction.
Ishiyama’s research team used transmission electron microscopy (TEM) to investigate the fine cellular structure of the BLB in the utricle, a balance-regulating organ of the inner ear. Two utricles were taken by autopsy from individuals with no vestibular or auditory disease. Five utricles were surgically extracted from patients with severe stage IV Ménière’s disease with profound hearing loss and intractable recurrent vertigo spells, who were undergoing surgery as curative treatment.
Microscopic examination revealed significant structural differences of the BLB within the utricle between individuals with and without Ménière’s disease. In the normal utricle samples, the VECs of the BLB contained numerous mitochondria and very few fluid-containing organelles called vesicles and vacuoles. The cells were connected by tight junctions to form a smooth, continuous lining, and were surrounded by a uniform membrane.
However, samples with confirmed Ménière’s disease showed varying degrees of structural changes within the VECs; while the VECs remained connected by tight junctions, an increased number of vesicles and vacuoles was found, which may cause swelling and degeneration of other organelles. In the most severe case, there was complete VEC necrosis, or cell death, and a severe thickening of the basal membrane surrounding the VECs.
The documentation of the cellular changes in the utricle of Ménière’s patients was the first of its kind and has important implications for future treatments. Ishiyama’s study concluded that the alteration and degeneration of the BLB likely contributes to fluid changes in the inner ear organs that regulate hearing and balance, thus causing the Ménière’s symptoms. Further scientific understanding of the specific cellular and molecular components affected by Ménière’s can lead to the development of new drug therapies that target the BLB to decrease vascular damage in the inner ear.
Gail Ishiyama, M.D., is a 2016 Emerging Research Grants recipient. Her grant was generously funded by The Estate of Howard F. Schum.
WE NEED YOUR HELP IN FUNDING THE EXCITING WORK OF HEARING AND BALANCE SCIENTISTS. DONATE TODAY TO HEARING HEALTH FOUNDATION AND SUPPORT GROUNDBREAKING RESEARCH: HHF.ORG/DONATE.
Therapies for Hearing Loss: What Is Being Tested?
By Pranav Parikh
Untreated hearing loss is linked to a lower quality of life, physical functionality, and communicative ability. The most common type of hearing loss, sensorineural, is often a result of damage to the delicate sensory hair cells in the inner ear. Because hair cell loss is irreversible, and hearing impairment therefore permanent, new treatment strategies are a welcome sign. In the July 2017 issue of Otology & Neurotology, Hearing Restoration Project (HRP) consortium member Ronna Hertzano, M.D., Ph.D., and Debara L. Tucci, M.D., a member of Hearing Health Foundation’s Council of Scientific Trustees (CST), along with Matthew Gordon Crowson, M.D., examined the field of emerging therapies for sensorineural hearing loss.
The team identified 22 active clinical drug trials in the U.S., and reviewed six potential therapies. Four use mechanisms to reduce oxidative stress believed to be involved in the inner ear cell death. Three of the therapeutic molecules being tested—D-methionine, N-acetylcysteine (NAC), and glutathione peroxidase mimicry (ebselen)—act as antioxidants to mop up free radicals caused by noise or other trauma to the inner ear. (For more about D-methionine, see page 11.) The fourth, sodium thiosulfate, is a chemical found to counteract the ototoxic effects of chemotherapy drugs.
The fifth approach is to manipulate the “cell death cascade.” This occurs when cells endure significant stress or injury, leading to the release of free radicals and changes in pH and protein that then kill the cell. Since hair cells do not regenerate like other cells, the cell death cascade causes permanent hearing loss. A trial is underway to make the cochlear neuroepithelium (inner ear tissue) more resilient to cell death signaling, using an inhibitor called AM-111 to block the chain of events leading to cell death. Finally, the sixth approach is a novel hair cell replacement therapy using the gene Atoh1, known to be a vital regulator of hair cell regeneration, causing cells to differentiate (change) into hair cells. Using mouse models, it has been shown that if Atoh1 is blocked, hair cell differentiation does not occur, and if it is induced, hair cell formation occurs, at least in the ears of very young mice.
Drug delivery methods to the inner ear are also being investigated. In addition to orally, delivery methods include a topical ear gel, intravenous infusion, and, most revolutionarily, direct injection of viruses to deliver genes to the inner ear. And while many of the drugs had to overcome hurdles to reach late-phase clinical trials, questions about safety, efficacy, and side effects remain, in addition to whether animal model results translate to human biology.
HRP consortium member Ronna Hertzano, M.D., Ph.D. (far left), is an assistant professor at the University of Maryland School of Medicine. HHF CST member Debara L. Tucci, M.D., is a professor at Duke University Medical Center in North Carolina.
This article originally appeared in the Fall 2017 issue of Hearing Health magazine. Find it here, along with many other innovative research updates.
Empower the Hearing Restoration Project's life-changing research. If you are able, please make a contribution today.
Gaining Better Clarity of Neural Networks
By Pranav Parikh
The ear, just like any other organ in the human body, uses nerves to function properly. One of the most vital nerves that the ear uses is the cochlear nerve, which connects the inner ear to the brain, or more specifically to the tonotopically-based regions of the cochlear nuclear complex located in the brainstem. This nerve shares the same shape and design of most nerves in the body, with dendrites absorbing information from various sources, sending the signal down the axon of the nerve through action potentials, and terminating the signal in a synapse so the message can be spread. In order to allow for this process to occur expediently, the nerve encounters a process known as myelination (providing a myelin sheath to propagate a signal faster). This is done through a glial cell known as an oligodendrocyte. Oligodendrocytes form a layer of lipid (fat) and protein around the axon to provide insulation, thereby allowing for signals to be sent to the brain more efficiently.
The immunoreactivity of Olig2 was detected during postnatal day (PND) 0 to 7, which became weaker after PND 10. Before PND 7, the majority of Olig2-expressing cells were found within the modiolus at the basal cochlear turn, while a few cells were located peripherally to the DIC-PCTZ and in close proximity to the spiral lamina at the basal cochlea turn. After PND 7, Olig2-expressing cells were fully overlapped with the DIC-PCTZ within modiolus at the spiral lamina in the basal cochlea.
A team of scientists led by Dr. Zhengqing Hu, funded by Hearing Health Foundation through its Emerging Research Grants program (2010 & 2011) was able to analyze oligodendrocyte protein expression in the cochlear nerve of postnatal mice. Through the use of Differential Interference Contrast (DIC) microscopy, they were able to investigate the cochlear nerve at staggered postnatal days, meaning the period following birth.
Their findings indicate oligodendrocytes are found to migrate along with the transition zone between the central and peripheral nervous systems. As the fetus develops after birth, and myelination occurs in the nerves connecting to the brain, the oligodendrocyte protein marker Oligo2 was observed. This could mean loss of hearing function could be connected to unmyelinated axons. There are many other neurodegenerative autoimmune diseases, such as multiple sclerosis, caused by demyelination, and hearing loss could potentially be added to that list. Dr. Hu’s work improves clarity of the neural network connecting the inner ear and the brain.
Zhengqing Hu, M.D., Ph.D. , is a 2010 and 2011 Emerging Research Grants recipient. Hu's research was published by Otolaryngology-Head and Neck Surgery on July 11, 2017.
We need your help supporting innovative hearing and balance science through our Emerging Research Grants program. Please make a contribution today.
New Clues to Sound Localization Issues in Fragile X Syndrome
By Pranav Parikh, Kathleen Wallace, and Elizabeth McCullagh, Ph.D.
Fragile X syndrome (FXS), the most common genetic form of autism, is characterized by impaired cognition, hyperactivity, seizures, attention deficits, and hypersensitivity to sensory stimuli, specifically auditory stimuli.
Individuals with FXS also experience difficulty with determining the source of a sound, known as sound localization. Sound localization is essential for listening in the presence of background noise such as a noisy classroom. The ability to localize sound properly is due to precise excitatory and inhibitory inputs to areas of the brain. 2016 Emerging Research Grants recipient Elizabeth McCullagh, Ph.D., and colleagues hypothesize that the auditory symptoms seen in FXS, specifically issues with sound localization, are due to an overall imbalance of excitatory and inhibitory synaptic input in these brain areas.
Investigators compared number and size of synaptic structures in different areas of the brainstem responsible for sound localization for several inhibitory neurotransmitters (glycine and GABA) and the primary excitatory neurotransmitter (glutamate) in a mouse model of FXS with a control group. The areas of the brainstem responsible for sound localization are connected to one another in a frequency-specific manner, with low frequency sounds stimulating similar areas and the same for high frequency. It was found that most areas of the brainstem examined did not have changes in number or size of structures, but one area—the medial nucleus of the trapezoid body (MNTB)—had alterations to inhibitory inputs that were specific to the frequency encoded by that region. Glycinergic inhibition was decreased in the high frequency region of MNTB, while GABAergic inhibition was decreased in the low frequency region.
The study by McCullagh and team in The Journal of Comparative Neurology is the first to explore alterations in glycinergic inhibition in the auditory brainstem of FXS mice. Due to the well-characterized functional roles of excitatory and inhibitory neurotransmitters in the auditory brainstem, the sound localization pathway is an ideal circuit to measure the sensory alterations of FXS. Given the findings in this study, further knowledge of the alterations in the lower auditory areas, such as the tonotopic differences in inhibition to the MNTB, may be necessary to better understand the altered sound processing found in those with FXS.
Elizabeth McCullagh, Ph.D., was a 2016 Emerging Research Grants scientist and a General Grand Chapter Royal Arch Masons International award recipient. For more, see “Tonotopic alterations in inhibitory input to the medial nucleus of the trapezoid body in a mouse model of Fragile X syndrome” in The Journal of Comparative Neurology.
We need your help supporting innovative hearing and balance science through our Emerging Research Grants program. Please make a contribution today.
New Study Suggests Serotonin May Worsen Tinnitus
Millions of people suffer from the constant sensation of ringing or buzzing in the ears known as tinnitus, creating constant irritation for some and severe anxiety for others. New research by scientists at OHSU shows why a common antidepressant medication may actually worsen the condition.