Findings suggest several FDA-approved drugs, such as a common diabetes medication and anesthetics, could protect from noise-induced hearing loss.
By the University of Maryland School of Medicine
A growing number of people are suffering from hearing loss due to exposure to loud noises from heavy machinery, concerts, or explosions. As a result, scientists have been working to understand the mechanism behind how the damage to hearing actually occurs.
Now, a team led by researchers at the University of Maryland School of Medicine (UMSOM) has published an online interactive atlas representing the changes in the levels of RNA made in the different cell types of ears of mice, after damage due to loud noise. These changes in RNA levels are known as changes in "gene expression."
Once they determined the larger trends in gene expression following the damage, the UMSOM scientists then searched a database of FDA-approved drugs to find those that are known to produce opposite patterns of those caused by the noise. From this analysis, the research teams identified a handful of drug candidates that may be able to prevent or treat the damage, and ultimately preserve hearing.
Their analysis was published in Cell Reports on September 28.
"As an otolaryngologist surgeon-scientist, I see patients with hearing loss due to age or noise damage, and I want to be able to help prevent or even reverse the damage to their hearing," says study leader Ronna Hertzano, M.D., Ph.D., a professor of otorhinolaryngology–head & neck surgery, anatomy, and neurobiology at UMSOM and an affiliate member of UMSOM's Institute for Genome Sciences. "Our extended analysis gives us very specific avenues to follow up on in future studies, as well as provides an encyclopedia that other researchers can use as a resource to study hearing loss."
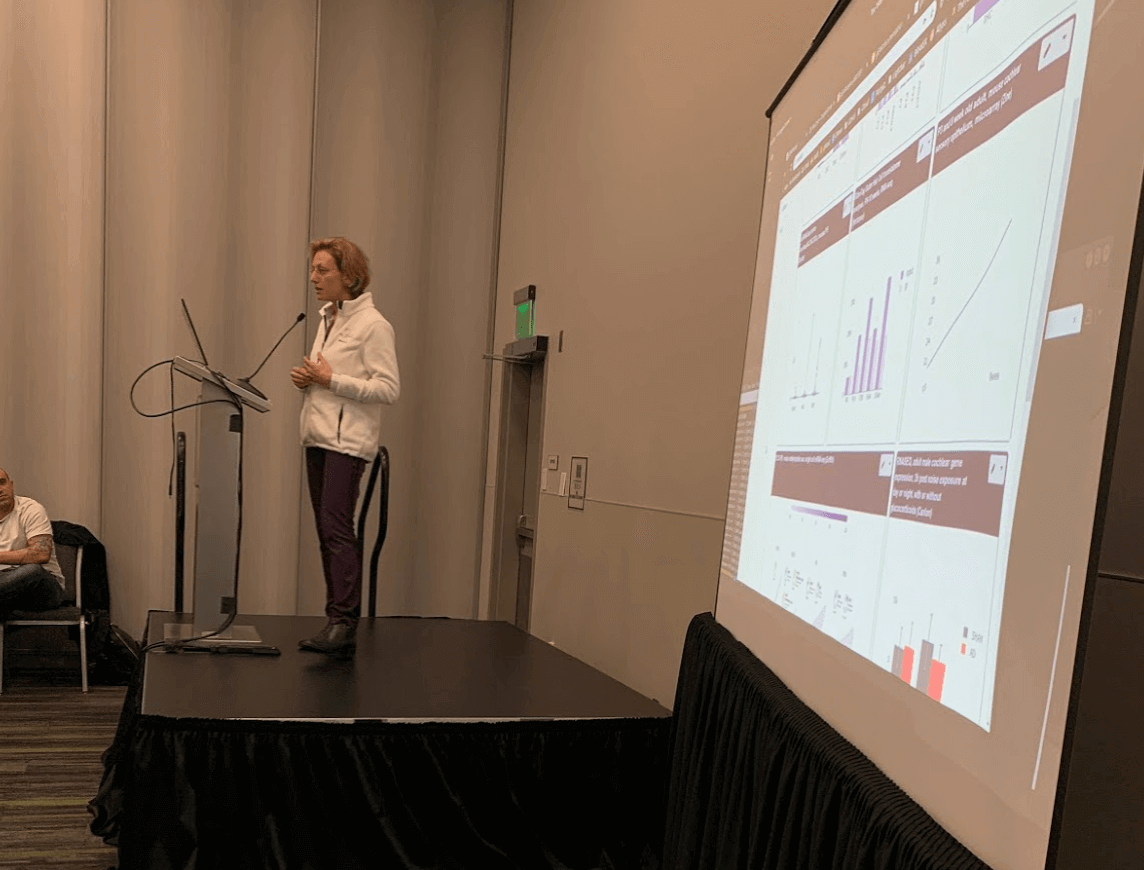
The team added their newest data on noise-induced hearing loss to the gEAR—the Gene Expression Analysis Resource—a tool developed by her laboratory that allows researchers not trained in informatics to browse gene expression data (published earlier this summer).
Hearing Restoration Project member and Emerging Research Grants alumnus Ronna Hertzano, M.D., Ph.D., demonstrates the gEAR, the data visualization tool she developed.
Hertzano explains that the inner ear resembles the shell of a snail, with separate fluid compartments and sensory cells along its entire length. The ear functions like a battery with a gradient of ions between the fluid compartments that is generated by the side wall of the shell by adding in potassium. The sensory cells detect sound and then communicate with the neurons that interact with the brain to interpret the signal. The sensory cells are surrounded by support cells. The inner ear also has resident immune cells to protect it from infection.
Research supervisor Beatrice Milon, Ph.D., in Hertzano's laboratory initially did an analysis on the sensory cells and the support cells of the ear in mice. She collected data on the changes in gene expression from before and after noise damage. After making their study known to other researchers in their field, the team heard from scientists at Decibel Therapeutics (led by Joe Burns, Ph.D.) and the Karolinska Institute (led by Barbara Canlon, Ph.D.), who had the gene expression data from the inner ear's neurons, side wall and immune cells from before and after noise damage. The teams then combined the datasets and performed their analysis.
The bioinformatic analyses were led by Eldad Shulman from the lab of Ran Elkon, Ph.D., Tel Aviv University, a bioinformatics expert that has been working collaboratively with Hertzano now for over two decades. Together, they leverage advanced computational techniques and combine them with biological insights to analyze and interpret data, providing impactful insights to the hearing research field.
Hertzano says it was so important that they looked at a cell-specific level, rather than looking at the entire ear, because they found that most of the gene expression changes were specific to only one or two cell types.
"We expected the subset of neurons typically sensitive to noise and aging, to have ‘bad’ changes in genes, so that we could counter them with drugs, but there was no such thing," Hertzano says. "On the contrary, we found that the subset of neurons that are resistant to noise trauma turn on a program that protects them while the very sensitive neurons had little change in gene expression. We are currently looking into approaches to induce the protective changes in the noise-sensitive neurons to prevent their loss from noise and aging."
In another example, the researchers found that only one out of the four types of immune cells detected showed major differences in gene expression.
Additionally, immune-related genes were turned up in all cell types of the inner ear after noise damage with many of them controlled by two key regulators.
The research team took the overall gene expression trends and plugged them into DrugCentral, a database of known molecular responses to FDA-approved drugs, specifically searching for changes that would be opposite of those happening in the noise-damaged cells. They identified the diabetes drug metformin as a potential candidate, as well as some inhaled anesthetic medications used in surgeries and other medications.
"Hearing aids and cochlear implants are used to alleviate hearing loss, however, there are no therapies available to prevent or treat hearing loss," says E. Albert Reece, M.D., Ph.D., MBA, the executive vice president for medical affairs, UM Baltimore, and the John Z. and Akiko K. Bowers Distinguished Professor, and Dean, UMSOM. "The studies that follow up on these findings may eventually lead to medications to prevent occupational noise-induced hearing loss, for example in factory workers, and to changes in standardizing anesthesia protocols for ear surgery, particularly in hearing preservation procedures."
This work was funded in part by Hearing Health Foundation’s Hearing Restoration Project.
This originally appeared on the University of Maryland School of Medicine website. HHF’s Hearing Restoration Project (HRP) member Ronna Hertzano, M.D., Ph.D., is a 2009–10 Emerging Research Grants (ERG) scientist. Hertzano and HRP scientific director Lisa Goodrich, Ph.D., recently hosted a webinar on hair cell regeneration; please see hhf.org/webinar to view the subtitled recording. For more, see hhf.org/hrp and hhf.org/erg.






The internship last summer provided my first real chance to step into hearing science and learn the experimental side of speech perception under the tutelage of a senior researcher.