By Amanda Janesick, Ph.D., and Nesrine Benkafadar, Ph.D.
Chickens are an important animal model for hearing restoration research because they are naturally capable of regenerating cochlear hair cells. This fact is known for many years, but the mechanisms that initiate and control this regenerative ability are unknown. The funding provided by Hearing Health Foundation through the Hearing Restoration Project (HRP) has helped the development of a new research program in the laboratory of Stefan Heller, Ph.D., at Stanford University focusing on chicken hair cell regeneration. Several years after its inception, this research is now bearing fruit.
In a series of two high-profile publications in the journal Cell Reports, a research team led by postdoctoral fellows Amanda Janesick, Ph.D., and Nesrine Benkafadar, Ph.D., reported several novel findings that lay the foundation for in-depth characterization of the cellular signaling that triggers and controls cochlear hair cell regeneration in birds. The work uses a recently developed technology called single-cell RNA-sequencing. This technology provides insight into changes in the activity of all genes as cochlear cells respond to hair cell damage and death.
To properly leverage single-cell RNA-sequencing technology, Janesick developed a new strategy to injure the chicken cochlea. The goal was to damage all hair cells “at once,” which is usually impossible with sound exposure or systemic injection of an ototoxic drug. However, using a surgical approach to the chicken cochlea, it is possible to inject a tiny amount of the drug sisomicin into the inner ear, causing all auditory hair cells to disappear within 24 hours.
Five days after ototoxic damage, the chicken regenerates sensory hair cells and neurons (green). Dead hair cell "ghosts" (red) float above the regenerating cells. Nuclei are marked in blue. Credit; Amanda Janesick, Ph.D.
Benkafadar took a closer look at the hair cells using single-cell RNA-sequencing. She found that cochlear hair cells, when damaged and stressed by exposure to sisomicin, activate a particular set of genes related to cellular stress. Her data show that hair cells “fight” to stay alive by invoking cellular repair mechanisms.
Benkafadar further discovered a mechanism that gets activated to cause hair cells to destroy their genetic material and subsequently disintegrate. This process, called programmed cell death, is well known and is generally a safety mechanism to prevent stressed cells from harming the body.
In the chicken cochlea, she found that hair cells “hang in there” for at least 16 hours before they dissolve and fall apart. During these 16 hours, the cells communicate with the remaining cells in the cochlea, the supporting cells. The researchers hypothesize that such signals ultimately instruct the supporting cells to commence the hair cell regeneration process. Ongoing work focuses on identifying the signals.
In total, this research created a comprehensive inventory of more than 15,000 individual genes activated and deactivated genes throughout the first 24 hours of hair cell demise. This rich source of important data is publicly available to the research community and will become the basis for future work that is now directly aimed at finding the relevant genes that initiate and control hair cell regeneration in birds.
In parallel, the HRP consortium is already working on comparing the identified genes with their mammalian equivalents, which will accelerate the translation of the findings towards future treatments.
A second aspect of the work that came from Janesick’s “inventorizing” of the different cell groups of the chicken cochlea was the identification of many new marker genes for the different cochlear cell types. Two surprises resulted:
1) A new hair cell type was discovered in the chicken inner ear. This finding will spur research toward uncovering the physiological function of this novel hair cell type.
2) The work identified two distinct groups of supporting cells, which are the stem cells that give rise to new hair cells after damage. Interestingly, the two groups are spatially distinct, corresponding to two different places in the cochlea that utilize different mechanisms for hair cell regeneration. One mechanism is based on cell division, which is the desired strategy that the Heller lab focuses on for developing future therapeutics to regenerate hair cells in the mammalian cochlea.
“The HRP provided the funding to get this study started in an environment where it was impossible to obtain support for this work from the NIH,” Heller says. “I hope that our data and the published results from the HRP consortium work will convince the National Institutes of Health to support the necessary follow-up studies that will hopefully lead to new treatments for patients.”
Amanda Janesick, Ph.D., (left, top) and Nesrine Benkafadar, Ph.D., are postdoctoral fellows in the laboratory of Stefan Heller, Ph.D., a member of HHF’s Hearing Restoration Project and a professor of otolaryngology–head & neck surgery at Stanford University, California. “Transcriptomic Characterization of Dying Hair Cells in the Avian Cochlea” and “Cell Type Identity of the Avian Cochlea” appeared in the March 23, 2021, issue of Cell Reports. For more, see hhf.org/hrp.





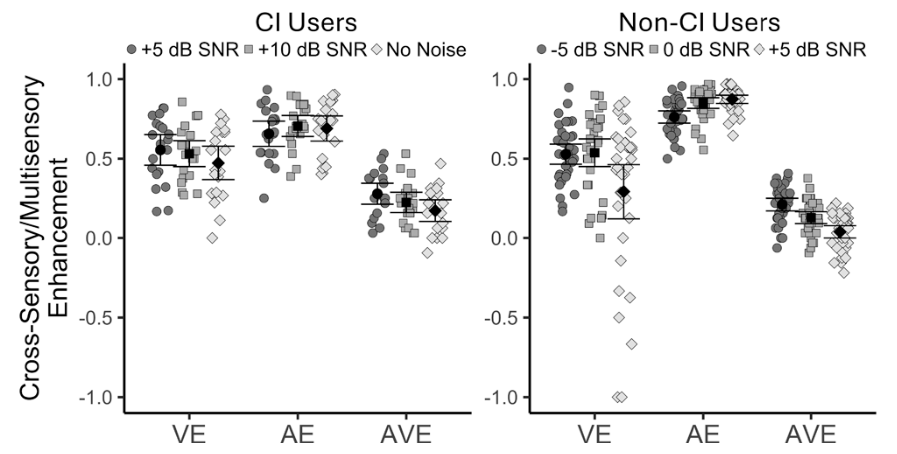

The internship last summer provided my first real chance to step into hearing science and learn the experimental side of speech perception under the tutelage of a senior researcher.