By Vijaya Prakash Krishnan Muthaiah, Ph.D.
Tinnitus is a phantom sensation of hearing sound without an external source which may occur with or without hearing loss. It is not a disease but rather a symptom of an underlying condition. However, to date there are no clear, definitive management recommendations for tinnitus, whether non-pharmacological or pharmacological. This is mainly due to a poor understanding of the pathomechanism (causes) of tinnitus. An imbalance in the excitation and inhibition of neurotransmitters is implicated, but more research is warranted.
To better understand the pathomechanism, many studies examine physiological changes due to an acute or chronic exposure to high levels of sound or using salicylate. To have more insight, many other studies of tinnitus, target concentrations levels of specific molecules using indirect, noninvasive methods in humans and using both invasive and noninvasive methods in animals. These methods have identified the neurochemistry underlying physiological abnormalities induced by tinnitus, and have begun to probe the many molecular mechanisms that occur along the auditory pathway.
This is where the use of mass spectrometry imaging (MSI) has been a powerful new approach in tinnitus research. MSI combines the highly specific in vivo detection of neurotransmitters with the whole-section visualization. This approach allows direct mapping of molecules throughout tissue sections; this is in contrast to other analyses of specific stereotaxic coordinate of brain tissue using ex vivo (such as high-performance liquid chromatography) or in vivo methods (such as microdialysis or fast scanning cyclic voltammetry). Therefore, this MSI method of global analysis of neurotransmitters will be a promising tool by providing an anatomical correlation of neurotransmitter levels with ultra-high resolution. The outcome of this approach will explain the physiological correlates that are being investigated in many basic and translational studies probing tinnitus mechanisms.
Also of note is that the direct link from an animal model of noise-induced tinnitus to the human pathophysiological response has not been perfect, with many mouse, rat, and guinea pig models having been investigated. The chinchilla model, however, has similarities to human hearing frequencies and how it transmits (conducts) sound from the ear to the brain.
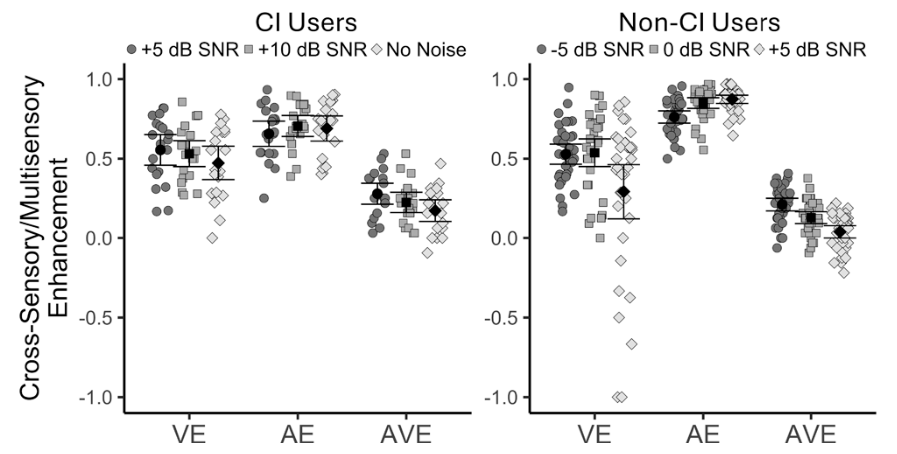
These intensity maps show the detected GABA (least to most intense on a gradation of blue to pink) in the primary centers of the brain’s auditory pathway—the auditory cortex, cochlear nucleus, and inferior colliculus—in the control animal compared with the chinchilla with blast-induced tinnitus. In the latter, GABA is clearly more varied in the auditory cortex and depleted in the inferior colliculus.
For our study in Experimental and Molecular Biology in April 2021, we produced relative quantitative imaging of the excitatory neurotransmitter glutamate and the inhibitory neurotransmitter GABA in the chinchilla central auditory neuraxis, which includes the auditory cortex, cochlear nuclei, and inferior colliculus, the primary centers of the auditory pathway.
The ultra-high resolution, quantitative imaging was performed using Fourier transform ion cyclotron resonance mass spectrometry. Our research provides insights about the homeostasis (state of equilibrium) of GABA/glutamate within whole-brain sections of the chinchilla in order to investigate the pathomechanism of blast-induced tinnitus.
The data obtained was formed into pixelated images of unique compounds, as shown in the figure highlighting the detection of the GABA molecule in sections of the brain containing the auditory cortex, cochlear nuclei, and inferior colliculus. After comparing the blast overpressure–exposed subject to a control, we observed that exposure to acute levels of noise disrupted GABA levels. Three days post-exposure, we saw a unilateral variance of the inhibitory GABA in the auditory cortex and a global depletion of GABA in the inferior colliculus, while levels of the excitatory glutamate stayed relatively constant.
Our results showing the depletion of GABA in the auditory cortex and inferior colliculus in the blast overpressure–exposed chinchilla model is in agreement with evidence of a GABA reduction observed utilizing electrophysiology and noninvasive measures in humans and rats. By adding this directly measured data, we strengthen the hypothesis that neurotransmitter disruption is linked to the persistence of tinnitus. In future studies by including more animals, at multiple time points, multiple exposures, and other molecular changes will be profiled to study tinnitus in the chinchilla model using these methodologies.
Vijaya Prakash Krishnan Muthaiah, Ph.D., is an assistant professor in the department of rehabilitation sciences at the University at Buffalo, the State University of New York. His 2019 Emerging Research Grant was generously funded by the General Grand Chapter Royal Arch Masons International.




I made one hat to solve problems, never imagining how many other adults and children would relate. It’s an honor to be able to give something back to the cochlear implant community that understands this journey so well.