Auditory brainstem responses (ABR) provide information about auditory function and hearing sensitivity. It tests auditory brainstem function in response to auditory (click) stimuli. The ABR has been widely used in the clinic for a range of purposes, from universal screening of newborn hearing to differential diagnosis of auditory neuropathy. Recently, the ABR has been shown to identify cochlear synaptopathy, a newly discovered hearing disorder resulting from even moderate noise exposure.
Cochlear synaptopathy does not refer to outer hair cell damage, but rather to swollen synapses between the inner hair cells and auditory nerve fibers; this produces secondary nerve injury predominantly in the low-spontaneous-rate, high-threshold nerve fibers. Because the nerve fibers responding to higher stimulus levels are disproportionately affected, animals with cochlear synaptopathy have typical wave I amplitude at or near thresholds but reduced wave I amplitude at higher stimulus levels.
As a result of this differential effect, the corresponding slope of the wave I growth function is also reduced. Since cochlear synaptopathy has been suggested as a mechanism underlying tinnitus, the ABR may serve as an objective biomarker for tinnitus in humans. Indeed, two studies found significantly reduced wave I amplitude in human tinnitus sufferers. This positive finding of reduced wave I amplitude is theoretically important because it establishes a link between cochlear neuropathy, which has been demonstrated in animals only, and tinnitus, which can be only subjectively reported by humans.
However, the prior research adopted stringent inclusion criteria, limited to relatively young (33 to 43 years old) tinnitus sufferers with typical audiograms. Because only 15 percent of tinnitus sufferers have typical hearing thresholds and the prevalence of tinnitus increases with age to 70 years, these stringent criteria would exclude up to 90 percent of the general tinnitus population. This context raises questions over whether the ABR wave I amplitude is sufficiently sensitive or specific to differentiate between the majority of human listeners with and without tinnitus.
A team led by 2016 Emerging Research Grants scientist Harrison Lin, M.D., measured the ABR in a diverse group of tinnitus subjects and also in a control group of non-tinnitus subjects that was matched to the tinnitus group in age and hearing loss. The hypothesis was that if cochlear synaptopathy played a major role in humans as conjectured by animal studies, then the ABR wave I amplitude or slope should be reduced in the tinnitus subjects.
A total of 43 human subjects, consisting of 21 with tinnitus and 22 without tinnitus, participated in the study, average age 44 +/- 24 years. Our results, published in the journal Brain Sciences in January 2022, showed that when compared with control subjects, tinnitus subjects did not show reduced ABR wave I amplitude or slope in either the entire group of 21 tinnitus subjects or a subset of tinnitus subjects with typical audiograms. Despite the small sample size and diverse tinnitus population, the present result suggests that the clinical utility of conventional ABR measurement is limited in detecting tinnitus in humans.
A 2016 Emerging Research Grants scientist generously funded by The Barbara Epstein Foundation, Harrison Lin, M.D., is a cochlear implant surgeon with University of California Irvine. He is a board-certified otolaryngologist and fellowship-trained in otology, neurotology, and skull base surgery. This summary is adapted from the paper in Brain Sciences.


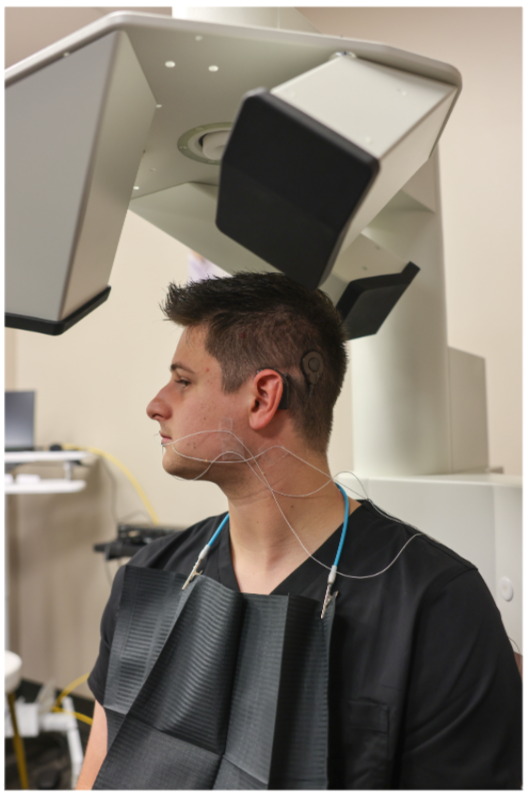



We are proud that Hearing Health Foundation-funded scientists are always well represented at Association for Research in Otolaryngology MidWinter Meeting.