By Daniel Fink, M.D.
The U.S. Preventive Services Task Force published its final recommendation on screening older adults for hearing loss on March 23, 2021, recommending against screening. As in its draft recommendation released a few months earlier (which I wrote about), the USPSTF “concludes that the current evidence is insufficient to assess the balance of benefits and harms of screening for hearing in older adults.” The USPSTF also published its Evidence Report and Systematic Review on which this decision is based. The decision is not surprising, given that the draft documents are little changed in their final form.
In March 2021, USPSTF announced it “concludes that the current evidence is insufficient to assess the balance of benefits and harms of screening for hearing in older adults.”
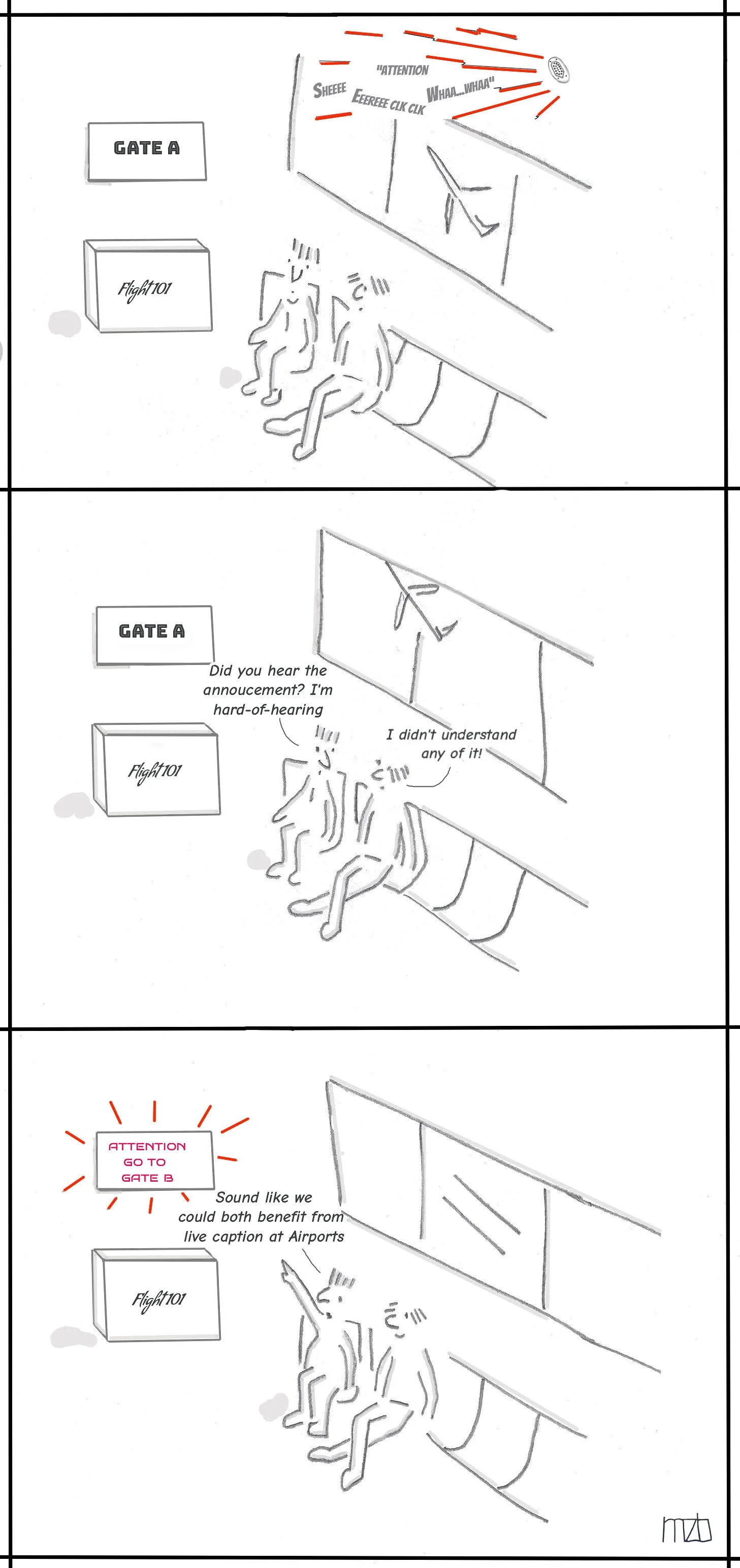
A 2017 publication from the U.S. Centers for Disease Control and Prevention found that almost 25 percent of American adults with hearing loss didn’t know they had any hearing problem. Hearing loss is not a benign condition. When left undiagnosed and untreated, it is associated in older adults with social isolation, depression, dementia, and falls. Approximately half of those over age 65 have hearing loss and this population is projected to increase to 77 million Americans by 2034.
Medical screening tests are used to identify asymptomatic patients in whom disease can be detected and prognosis altered through treatment. Hearing loss would appear to be an ideal condition for screening. Unfortunately, the research needed to show a benefit from screening for hearing loss hasn’t yet been done. Published studies show that hearing loss is common, but don’t show that treatment has any benefit.
Hearing health advocates and hearing health professionals are disappointed by the USPSTF recommendation. Fortunately, two recent medical journal editorials commenting on the recommendation suggest a way forward. A March 23–30, 2021, editorial in JAMA notes that “insufficient evidence does not mean insufficient benefit.” and discusses simple office-based screening techniques to detect hearing loss. It also suggests that physicians use their clinical judgment to identify patients with hearing loss who may benefit from sound amplification.
A March 23, 2021, editorial in JAMA Network Open discusses the fact that the treatment for hearing loss—hearing aids—is generally too expensive for most Americans, with fewer than 20 percent of those with hearing loss reporting hearing aid use. The authors mention proposed legislative solutions to this problem.
I think there are additional barriers to hearing aid use. There is the perceived stigma of hearing loss, and also the fact that many with hearing loss can’t afford hearing aids. Another problem is hearing aid non-use, also called “the hearing aid in the drawer” problem.
Regulation and technology may provide solutions. Federal law will soon officially allow the over-the-counter sale of less expensive personal sound amplification products (PSAPs), costing a few hundred rather than a few thousand dollars. PSAPs are available but for now, buyer beware, as they have yet to follow Food and Drug Administration safety guidelines—because these remain elusive. (Guidelines were due to be released in August 2020, but... COVID?)
Statistics show that the population of people in the U.S. impacted by hearing loss is on the rise, and that the vast majority are not utilizing treatment. Left to right: Half of adults ages 65+ in the U.S. currently have a hearing loss. By 2034, that age group will reach 77 million. 80% of Americans with hearing loss do not use hearing aids.
Once they are FDA regulated, OTCs will reduce the financial barriers for sound amplification products dramatically. And newer digital technologies may allow these off-the-shelf devices to help those with hearing loss achieve what they really want: understanding speech in everyday situations.
Hearing health professionals, families of those with hearing loss, those fitted with the proper hearing aids, and yes, those television ads, attest to the fact that hearing aids can transform the lives of those who need them. Unfortunately, as one of my mentors taught me, the plural of anecdotes isn’t data.
If sound amplification products are affordable or covered by insurance, and if they really help people understand speech in noisy situations, we can overcome the hearing-aid-in-the-drawer problem. For the stigma issue, as we’ve noted before, these days most everyone has something in their ears. Creative (cool) approaches to design can help reduce that limiting factor, and smaller in-the-canal hearing aids might eliminate it entirely.
With all these factors in mind, I hope that needed research can be done to show that screening for hearing loss and providing those needing them with appropriate sound amplification products really does have measurable benefits, and that the USPSTF will eventually change its recommendation.
Daniel Fink, M.D., is the founding chair of the Quiet Coalition, the interim chair of Quiet Communities' Health Advisory Council, and a former board member of the American Tinnitus Association. He serves as an expert consultant to the World Health Organization on its Make Listening Safe Program, and as a subject matter expert on noise and the public to the National Center for Environmental Health at the U.S. Centers for Disease Control and Prevention.






The internship last summer provided my first real chance to step into hearing science and learn the experimental side of speech perception under the tutelage of a senior researcher.