The sensory organs that allow us to walk, dance, and turn our heads without dizziness or loss of balance contain specialized synapses that process signals faster than any other in the human body.
By Jade Boyd
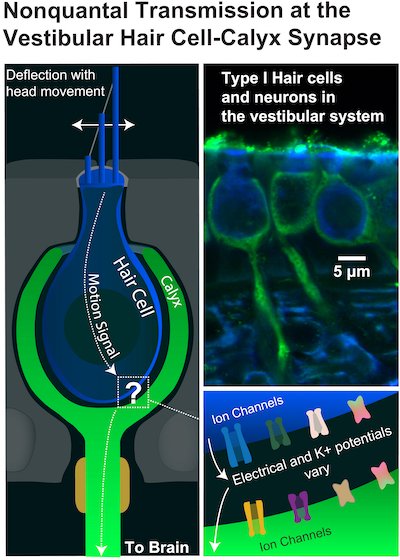
An illustration and microscopic images show the relationship between motion-sensing vestibular hair cells (blue) of the innermost ear and the cup-shaped “calyx” (green) structures of adjoining nerves that connect directly to the brain. The rapid flow of information through the synapses helps stabilize balance and vision in humans and many other animals. Researchers from Rice University, the University of Chicago and the University of Illinois Chicago created the first quantitative model that shows how potassium ions (K+) and electrical signals are transmitted across the synapses to rapidly deliver information to the brain. (Credit: Aravind Chenrayan Govindaraju/Rice University)
In a discovery more than 15 years in the making, a small group of neuroscientists, physicists and engineers from several institutions has unlocked the mechanism of the synapses, paving the way for research that could improve treatments for vertigo and balance disorders that affect as many as 1 in 3 Americans over age 40.
The study published in the Proceedings of the National Academy of Sciences in January 2023 describes the workings of “vestibular hair cell-calyx synapses,” which are found in organs of the innermost ear that sense head position and movements in different directions.
“Nobody fully understood how this synapse can be so fast, but we have shed light on the mystery,” says Robert Raphael, Ph.D., a Rice University bioengineer who co-authored the study with the University of Chicago’s Ruth Anne Eatock, Ph.D., the University of Illinois Chicago’s Anna Lysakowski, Ph.D., current Rice graduate student Aravind Chenrayan Govindaraju, and former Rice graduate student Imran Quraishi, M.D., Ph.D., now an assistant professor at Yale University.
Synapses are biological junctions where neurons can relay information to one another and other parts of the body. The human body contains hundreds of trillions of synapses, and almost all of them share information via quantal transmission, a form of chemical signaling via neurotransmitters that requires at least 0.5 milliseconds to send information across a synapse.
Prior experiments had shown a faster, “nonquantal” form of transmission occurs in vestibular hair cell-calyx synapses, the points where motion-sensing vestibular hair cells meet afferent neurons that connect directly to the brain. The new research explains how these synapses operate so quickly.
In each, a signal-receiving neuron surrounds the end of its partner hair cell with a large cuplike structure called a calyx. The calyx and hair cell remain separated by a tiny gap, or cleft, measuring just a few billionths of a meter.
“The vestibular calyx is a wonder of nature,” Lysakowski says. “Its large, cup-shaped structure is the only one of its kind in the entire nervous system. Structure and function are intimately related, and nature obviously devoted a great deal of energy to produce this structure. We’ve been trying to figure out its special purpose for a long time.”
From the ion channels expressed in hair cells and their associated calyces, the authors created the first computational model capable of quantitatively describing the nonquantal transmission of signals across this nanoscale gap. Simulating nonquantal transmission allowed the team to investigate what happens throughout the synaptic cleft, which is more extensive in vestibular synapses than other synapses.
“The mechanism turns out to be quite subtle, with dynamic interactions giving rise to fast and slow forms of nonquantal transmission,” Raphael says. “To understand all this, we made a biophysical model of the synapse based on its detailed anatomy and physiology.”
The model simulates the voltage response of the calyx to mechanical and electrical stimuli, tracking the flow of potassium ions through low-voltage-activated ion channels from presynaptic hair cells to the post-synaptic calyx.
Raphael says the model accurately predicted changes in potassium in the synaptic cleft, providing key new insights about changes in electrical potential that are responsible for the fast component of nonquantal transmission; explained how nonquantal transmission alone could trigger action potentials in the postsynaptic neuron; and showed how both fast and slow transmission depend on the close and extensive cup formed by the calyx on the hair cell.
“The key capability was the ability to predict the potassium level and electrical potential at every location within the cleft,” Eatock says. “This allowed the team to illustrate that the size and speed of nonquantal transmission depend on the novel structure of the calyx. The study demonstrates the power of engineering approaches to elucidate fundamental biological mechanisms, one of the important but sometimes overlooked goals of bioengineering research.”
Quraishi began constructing the model and collaborating with Eatock in the mid-2000s when he was a graduate student in Raphael’s research group and she was on the faculty of Baylor College of Medicine, just a few blocks from Rice in Houston’s Texas Medical Center.
Quraishi’s first version of the model captured important features of the synapse, but he says gaps in “our knowledge of the specific potassium channels and other components that make up the model was too limited to claim it was entirely accurate.”
Since then, Eatock, Lysakowski, and others discovered ion channels in the calyx that transformed scientists’ understanding of how ionic currents flow across hair cell and calyx membranes.
“The unfinished work had weighed on me,” Qurashi says, and he was both relieved and excited when Govindaraju, a Ph.D. student in applied physics, joined Raphael’s lab and resumed work on the model in 2018.
“By the time I started on the project, more data supported nonquantal transmission,” Govindaraju says. “But the mechanism, especially that of fast transmission, was unclear. Building the model has given us a better understanding of the interplay and purpose of different ion channels, the calyx structure and dynamic changes in potassium and electric potential in the synaptic cleft.”
“One of my very first grants was to develop a model of ion transport in the inner ear,” Raphael says. “It is always satisfying to achieve a unified mathematical model of a complex physiological process. For the past 30 years—since the original observation of nonquantal transmission—scientists have wondered, ‘Why is this synapse so fast?’ and, ‘Is the transmission speed related to the unique calyx structure?’ We have provided answers to both questions.”
Raphael says the link between the structure and function of the calyx “is an example of how evolution drives morphological specialization. A compelling argument can be made that once animals emerged from the sea and began to move on land, swing in trees, and fly, there were increased demands on the vestibular system to rapidly inform the brain about the position of the head in space. And at this point the calyx appeared.”
The model opens the door for a deeper exploration of information processing in vestibular synapses, he says, including research into the unique interactions between quantal and nonquantal transmission.
Raphael adds that the model could also be a powerful tool for researchers who study electrical transmission in other parts of the nervous system, and he hopes it will aid those who design vestibular implants, neuroprosthetic devices that can restore function to those who have lost their balance.
This originally appeared on the Rice University Office of Public Affairs website. A 2022 Emerging Research Grants (ERG) scientist, Robert Raphael, Ph.D., is an associate professor of bioengineering in Rice’s George R. Brown School of Engineering. He is also a 2007 ERG scientist. A former member of HHF’s board of directors and a 1987–88 and 1994 ERG scientist, Ruth Anne Eatock, Ph.D., is a professor of neurobiology at the University of Chicago.




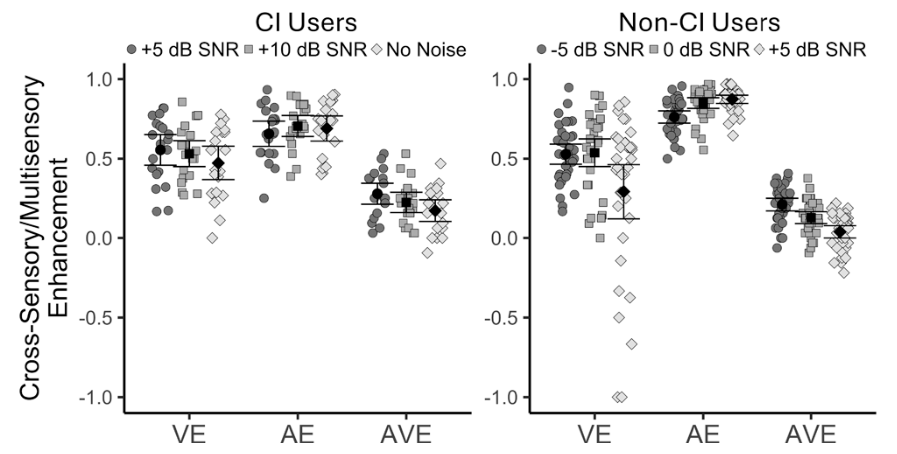
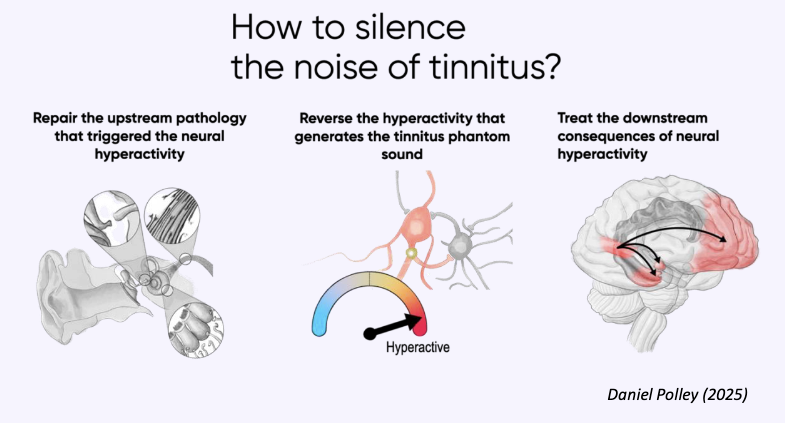

Because noise-canceling earbuds are so comfortable and block everything out, people wear them for three, four, five hours straight without realizing the cumulative effect on their ears.