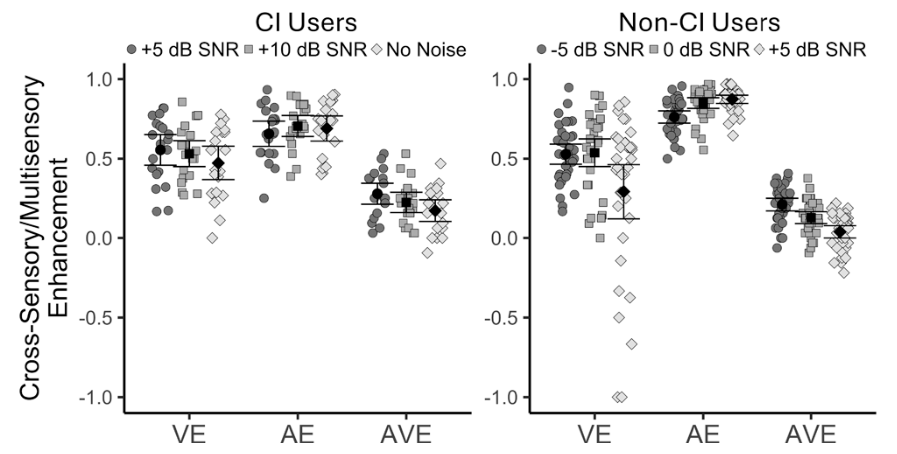
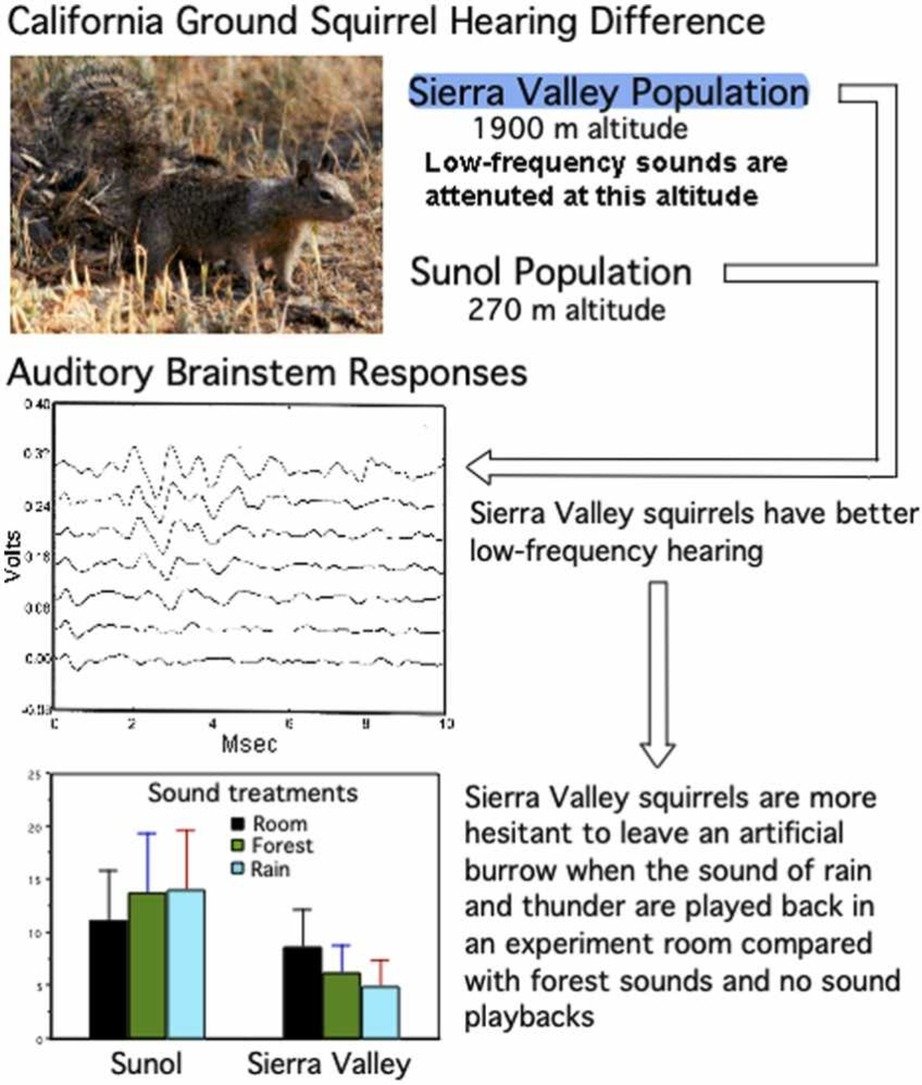
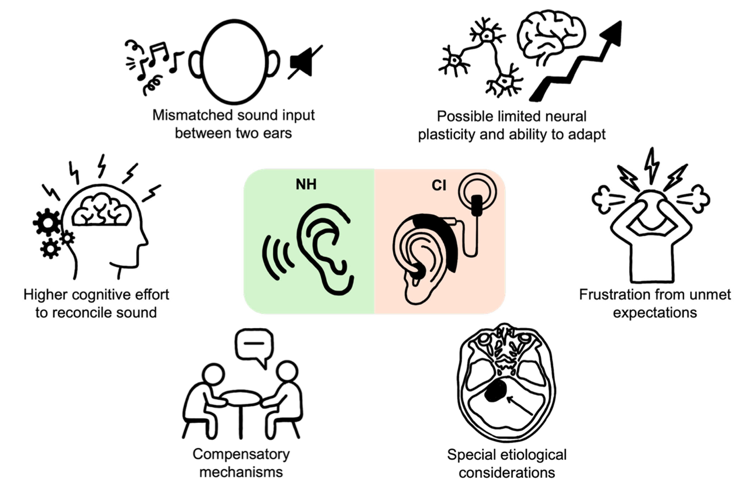
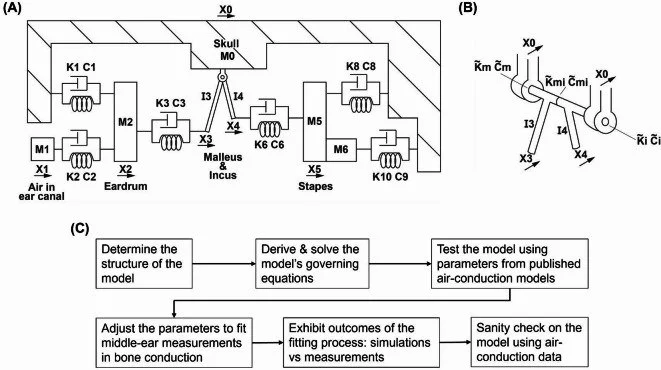
Seed Money for Hearing & Balance Researchers
Through the Emerging Research Grants (ERG) program, Hearing Health Foundation (HHF) provides seed money to researchers working on the entire spectrum of hearing research and balance research, including many underfunded areas of otology. The ERG program has since 1958 played a foundational role in the careers of many academic researchers and clinicians in otolaryngology and related hearing and balance fields. As the program’s remit is so broad, HHF solicits applications for ERG awards under a number of opportunity types.
The ERG program offers three award types:
Elizabeth M. Keithley, Ph.D. Early Stage Investigator Awards (EMKESIA): This grant opportunity supports projects across the broadest spectrum of hearing research and balance research. Applicants must be early stage investigators and fulfill certain additional eligibility requirements. These awards are for up to $50,000 per year.
Focused Discovery Awards (HHF-FDA): These are dedicated topic-specific grant opportunities open to eligible investigators at any career stage. Available grant opportunities vary by year. Please see the current Request for Proposals for a list of award topics for that application cycle. These awards are for up to $50,000 per year.
Expanded Discovery Awards (HHF-EDA): These are dedicated topic-specific grant opportunities open to eligible investigators at any career stage. Available grant opportunities vary by year. Please see the current Request for Proposals for a list of award topics for that application cycle. These awards are for up to $100,000 per year.
ERG awards are made for one year in the first instance and are renewable for a second year. The total two-year award amount is $100,000 for EMKESIA and HHF-FDA grants, and $200,000 for HHF-EDA grants.
The program is governed by the Council of Scientific Trustees, which is comprised of senior researchers and physicians from across the nation who assess each application for scientific merit and relevance. The ERG program is particularly targeted to early career researchers, but has funding streams open to researchers at all career stages.
The ERG program is a competitive process that awards grants to only the most promising investigators. Recipients are exceptionally well-positioned to win future grants from the National Institutes of Health (NIH) and other federal research funders, leading to dramatic innovations in the field. In fact, ERG alumni have gone on to be awarded an average $59 of additional federal funding for every dollar of investment through ERG (2002–present).
HHF welcomes applications from scientists who are based at research institutions (higher education, government, and/or non-profit) located in the U.S. and who hold an Au.D., M.D., Ph.D. or equivalent. Please see the full Policy on Emerging Research Grants for all eligibility information.
Applying for a Grant
2016 Emerging Research Grantee Elizabeth McCullagh, Ph.D. (front), and colleague Achim Klug, Ph.D., University of Colorado
Applications for the 2026–2027 cycle are no longer being accepted. The next request for proposals will be published in October 2026. You may sign up for grant alert emails by clicking the button below.
Timeline:
The Request for Proposals is published in mid-October each year.
LOIs are due in December.
Invited applicants must submit full proposals by April.
Applicants are notified in August.
The award period is October 1 through September 30 of the following calendar year. The award period start/end dates cannot be changed.
For more information and specific deadlines for the current application cycle, see the Application Page. Email grants@hhf.org with questions.
“HHF plays a seminal role in launching the independent careers of many scientists in hearing research. The grants awarded by HHF provide the ‘seed’ support needed to obtain preliminary data that enables a grantee to apply for and get a larger grant award to expand their research resources and efforts.”
Meet Our Emerging Research Grants Scientists
Here are our 2026 first-year and second-year ERG scientists. Learn more about their research below.
Council of Scientific Trustees
The Council of Scientific Trustees is the governing body of our Emerging Research Grants program. The CST comprises senior national researchers and physicians who review each application for scientific merit and program relevance.