Following a rigorous review process, our Scientific Review Committee and Council of Scientific Trustees, comprised of senior expert scientists and physicians from across the US, have chosen fourteen especially meritorious projects to fund, covering a broad range of hearing and balance science.
Very High-Frequency Hearing Loss and Tinnitus: Is There a Link?
With central inhibition lowered, signals that are typically dampened are able to be perceived, potentially resulting in tinnitus. Our paper also showed the utility of measuring central inhibition through cortical auditory evoked potentials (CAEPs), which are electrical responses in the brain that reveal levels of central inhibition.
Stability in an Unstable World
By studying the mouse brain, Balmer and Trussell have now mapped the direct and indirect circuits that carry sensory information to the vestibular cerebellum. Both types of input activate cells within the vestibular cerebellum called unipolar brush cells (UBCs).
Improved TMC1 Gene Therapy Restores Hearing and Balance in Mice
Half of all inner ear disorders, which have a negative impact on hearing and/or balance, are caused by genetic mutations. A study published in January 2019 in Nature Communications demonstrates the effectiveness of a gene therapy targeting one specific gene mutation, TMC1 (transmembrane channel-like 1).
Single-Cell RNA Sequencing Reveals More Clues for Hair Cell Regeneration
To study the genetic program of hair cell regeneration in zebrafish, we sequenced the RNA of individual cells within neuromasts, allowing us to classify cell types based on their gene expression signature.
Hearing Restoration Project Scientific Director to Lead University’s Research Enterprise
Peter Barr-Gillespie, Ph.D., will be Oregon Health & Science University’s (OHSU) first chief research officer and executive vice president, effective Jan. 1, 2019. Barr-Gillespie has served as interim senior vice president for research at OHSU since 2017.
First Study to Examine Cognitive Development in Deaf Babies Finds Differences Begin in Infancy
A noise injury worsens readily. For hyperacusis sufferers such as myself, quiet makes the condition better; noise makes it worse. Among sufferers this is indisputable, but medical practitioners bizarrely treat quiet as harmful.
Shared Knowledge Is Power
ARO provides auditory and vestibular researchers opportunities present their latest findings and engage in meaningful conversations with one another. If one scientist presents an idea to an audience of 100 scientists, she’s just created the possibility for 100 new ideas will form.
New Insights into the Development of the Hair Cell Bundle
By Yishane Lee
Recent genetic studies have identified that the protein Ripor2 (formerly known as Fam65b) is an important molecule for hearing. It localizes to the stereocilia of auditory hair cells and causes deafness when mutations disrupt its function.
In a study published in the Journal of Molecular Medicine in November 2018, Oscar Diaz-Horta, Ph.D., a 2017 Emerging Research Grants (ERG) scientist, and colleagues further show the role the protein plays by demonstrating how it interacts with other proteins during the development of the hair cell bundle. The team found that the absence of Ripor2 changes the orientation of the hair cell bundle, which in turn affects hearing ability.
Ripor2 interacts with Myh9, a protein encoded by a known deafness gene, and Myh9 is expressed in the hair cell bundle stereocilia as well as its kinocilia (apices). The team found that the absence of Ripor2 means that Myh9 is low in abundance. In the study, Ripor2-deficient mice developed hair cell bundles with atypically localized kinocilia and reduced abundance of a phosphorylated form of Myh9. (Phosphorylation is a cellular process critical for protein function.)
Another specific kinociliary protein, acetylated alpha tubulin, helps stabilize cell structures. The researchers found it is also reduced in the absence of Ripor2.
The study concludes that Ripor2 deficiency affects the abundance and/or role of proteins in stereocilia and kinocilia, which negatively affects the structure and function of the auditory hair cell bundle. These newly detailed molecular aspects of hearing will help to better understand how, when these molecular actions are disrupted, hearing loss occurs.
A 2017 ERG scientist funded by the Children’s Hearing Institute (CHI), Oscar Diaz-Horta, Ph.D., was an assistant scientist in the department of human genetics at the University of Miami. He passed away suddenly in August 2018, while this paper was in production. HHF and CHI both send our deepest condolences to Diaz-Horta’s family and colleagues.
We need your help supporting innovative hearing and balance science through our Emerging Research Grants program. Please make a contribution today.
Disrupted Nerve Cell Function and Tinnitus
By Xiping Zhan, Ph.D.
Tinnitus is a condition in which one hears a ringing and/or buzzing sound in the ear without an external sound source, and as a chronic condition it can be associated with depression, anxiety, and stress. Tinnitus has been linked to hearing loss, with the majority of tinnitus cases occurring in the presence of hearing loss. For military service members and individuals who are constantly in an environment where loud noise is generated, it is a major health issue.
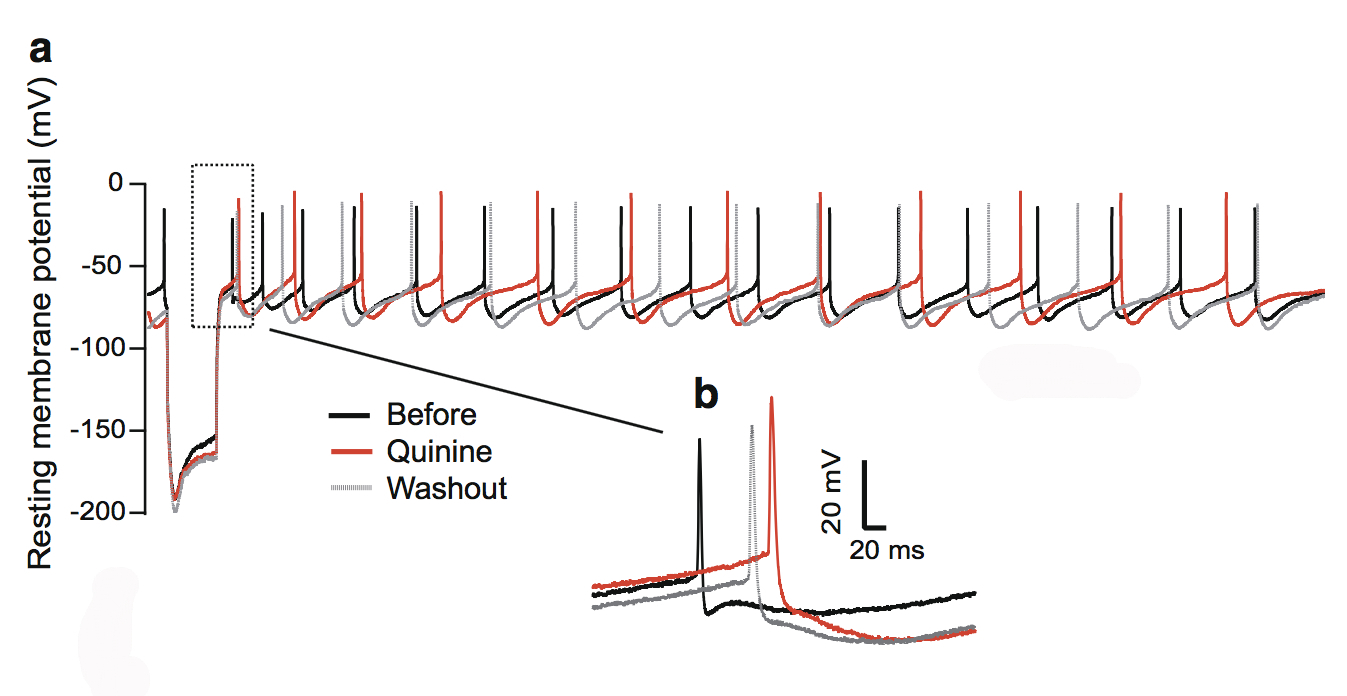
This figure shows the quinine effect on the physiology of dopaminergic neurons in the substantia nigra, a structure in the midbrain.
During this phantom ringing/buzzing sensation, neurons in the auditory cortex continue to fire in the absence of a sound source, or even after deafferentation following the loss of auditory hair cells. The underlying mechanisms of tinnitus are not yet known.
In our paper published in the journal Neurotoxicity Research in July 2018, my team and I examined chemical-induced tinnitus as a side effect of medication. Tinnitus patients who have chemical-induced tinnitus comprise a significant portion of all tinnitus sufferers, and approaching this type of tinnitus can help us to understand tinnitus in general.
We focused on quinine, an antimalarial drug that also causes hearing loss and tinnitus. We theorized this is due to the disruption of dopamine neurons rather than cochlear hair cells through the blockade of neuronal ion channels in the auditory system. We found that dopamine neurons are more sensitive than the hair cells or ganglion neurons in the auditory system. To a lesser extent, quinine also causes muscle reactions such as tremors and spasms (dystonia) and the loss of control over body movements (ataxia).
As dopaminergic neurons (nerve cells that produce the neurotransmitter dopamine) are implicated in playing a role in all of these diseases, we tested the toxicity of quinine on induced dopaminergic neurons derived from human pluripotent stem cells and isolated dopaminergic neurons from the mouse brain.
We found that quinine can affect the basic physiological function of dopamine neurons in humans and mice. Specifically, we found it can target and disturb the hyperpolarization-dependent ion channels in dopamine neurons. This toxicity of quinine may underlie the movement disorders and depression seen in quinine overdoses (cinchonism), and understanding this mechanism will help to learn how dopamine plays a role in tinnitus modulation.
A 2015 ERG scientist, Xiping Zhan, Ph.D., received the Les Paul Foundation Award for Tinnitus Research. He is an assistant professor of physiology and biophysics at Howard University in Washington, D.C. One figure from the paper appeared on the cover of the July 2018 issue of Neurotoxicity Research.
We need your help supporting innovative hearing and balance science through our Emerging Research Grants program. Please make a contribution today.