By James Dewey, Ph.D.
Audiophiles typically seek out sound systems that most accurately reproduce acoustic recordings and add minimal distortion. Interestingly, our sense of hearing does not work in quite such a transparent way. In fact, a considerable amount of distortion is introduced by the sensory hair cells within the inner ear.
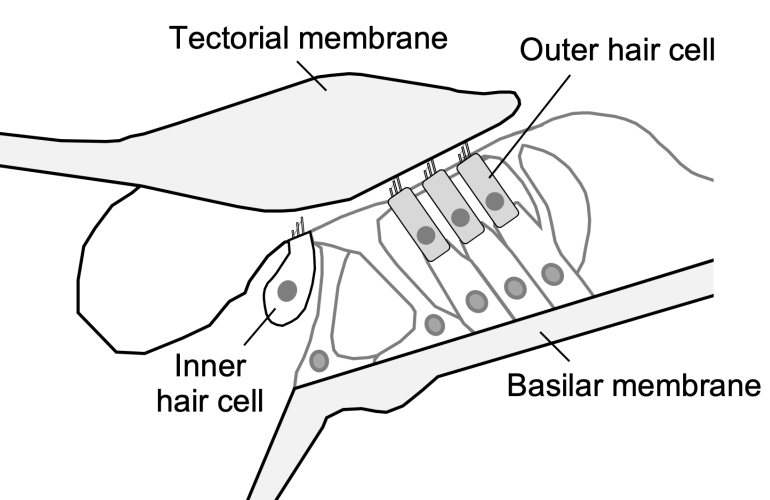
These cells—the inner and outer hair cells (see Fig. 1)—are responsible for converting sound-evoked vibrations of the surrounding structures into electrical signals that are transmitted to the brain. Distortion results from the fact that this conversion process is highly nonlinear (i.e., the relationship between vibration and the resulting electrical signal follows a sigmoidal curve and not a straight line).
Studying the distortions generated within the inner ear tells us how these signals may influence our perception of sound and also provides insight into the processes that are involved in basic sensory hair cell function. This function is very difficult to study in live ears, as the auditory sensory organ—the organ of Corti—is located deep within the temporal bone.
This schematic cross-section shows the organ of Corti within the inner ear, which contains the sensory inner and outer hair cells. Vibrations of the hair-like structures at the tops of the cells are converted to distorted electrical signals. Outer hair cells generate force in response to this electrical signal, causing distorted vibrations of the surrounding structures.
To better understand the distortion that sensory hair cells generate, I used an imaging technique called optical coherence tomography (OCT) to see into the mouse inner ear and measure vibrations of the hair cells in response to sound. While OCT is commonly used to examine the health of the human retina, it is also powerful enough to see through the bone encasing the inner ear in mice, allowing me to visualize how the hair cells vibrate. Since the outer hair cells, in particular, use their electrical signals to drive the generation of mechanical forces, any distortions in the electrical signals are converted into vibrations of the cells and surrounding structures, where they can be detected using OCT.
By presenting two tones to the mouse ear, one at a frequency f1 and the other at a frequency f2, I was able to study vibratory distortions at mathematically related frequencies like f2-f1 and 2f1-f2. These distortion frequencies are of interest as they theoretically depend on different properties of the underlying nonlinear function. While the 2f1-f2 distortion is often assumed to be largest and has been most widely studied, I actually found that f2-f1 was much larger for a wide variety of stimulus frequencies and amplitudes.
Remarkably, the distortions were sometimes as large as the responses at the frequencies of the stimulus tones themselves. The resolution and sensitivity of the OCT-based approach allowed me to characterize how the distortions were shaped as they were transmitted from the outer hair cells to the surrounding basilar and tectorial membranes, and how they traveled to different inner ear locations, just like the vibrations that were produced by sounds directly presented to the ear.
The findings, published in the Journal of the Acoustical Society of America Express Letters in November 2022, are important because they clarify how distortions are produced by the outer hair cells and how these additional signals may influence peripheral sound encoding.
Analysis of the distortions also allowed estimation of the nonlinear function that determines how the cells respond to sound, which so far has primarily been studied in vitro. Since the forces generated by outer hair cells serve to amplify the stimulation of the inner hair cells, which are the main communicators of auditory information to the brain, the findings also reveal aspects of the physical processes that are required for sensitive hearing.
A 2020 and 2023 Emerging Research Grants scientist, James Dewey, Ph.D., is an assistant professor of otolaryngology-head and neck surgery at the Keck School of Medicine of the University of Southern California.




I made one hat to solve problems, never imagining how many other adults and children would relate. It’s an honor to be able to give something back to the cochlear implant community that understands this journey so well.