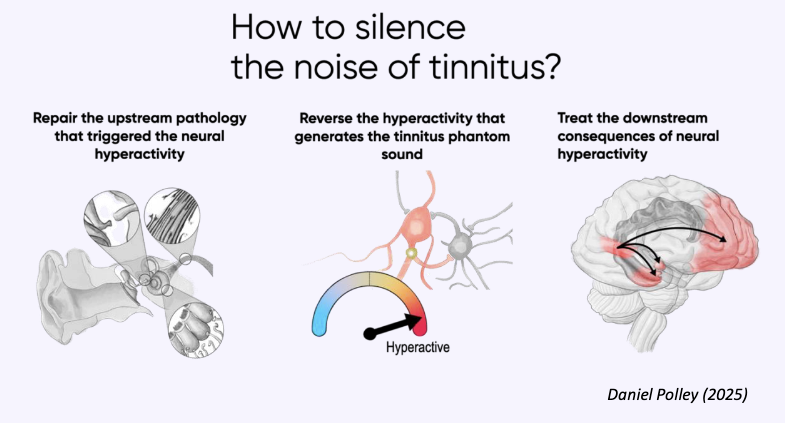
By Jennifer Resnik, Ph.D.
When the axons (nerve fibers) of neurons (nerve cells) are damaged or dysfunctional, the sensory information that is relayed from sensory organs, such as the ear, to the brain is reduced and limited—like dead pixels in a computer display. In the auditory nerve, it is type I spiral ganglion neurons (SGNs) that transmit virtually all signals from sensory hair cells in the cochlea to neurons in the auditory brainstem. These type I SGNs are the most vulnerable element in the inner ear, sensitive to environmental effects and aging.
Seminal work by M. Charles Liberman, Ph.D., and Sharon Kujawa, Ph.D., a decade ago revealed that acoustic overexposure can damage afferent cochlear neurons even when cochlear hair cells remain intact. Since then, work in over a dozen animal species has demonstrated a marked reduction of type I SGN functionality following exposure to ototoxic drugs or environmental noise. In humans, postmortem analysis of the inner ear shows that approximately 40 percent of SGN axons have already degenerated by age 50, well in advance of comparable losses in other inner ear cell types.
Even a small fraction of functional pixels would be sufficient to determine whether a computer was on or off. The difficulty would come in the recognition of a complex image. Eliminating as much as 95 percent of type I SGNs in mice has minimal effects on their ability to hear in quiet backgrounds. The challenge is hearing in noisy environments. In humans, evidence suggests that difficulty hearing in noisy, social settings—a symptom of hidden hearing loss—may reflect premature auditory nerve degeneration.
Cochlear neural degeneration (CND) in adult mice disrupts behavioral detection of tones in noise but not in silence.
In our study, published in the journal Neuron in March 2021, Daniel Polley, Ph.D., and I report finding deterioration in perception in noisy environments after inducing bilateral moderate auditory nerve degeneration in adult mice. We found that the resulting lesions produced surges of noisy, synchronized activity in the auditory cortex that preceded the mice’s failures to detect target sounds in noise.
Specifically, cochlear neuropathy produced distinct forms of cortical (brain) plasticity in both excitatory and inhibitory neurons that culminated in net hyperactivity, increased neural gain, and reduced adaptation to background noise. This pattern was not present in the typical-hearing mice, whose cortical responses maintained a typical balance of excitation and inhibition. In the typical-hearing mice, the lack of a target-evoked response predicted behavioral misses; in contrast, in mice after cochlear neuropathy, random surges of cortical activity before the tone was presented reliably predicted impending failures to detect sound—revealing a source of internal cortical noise underlying difficulties in the perception of external noise.
One encouraging finding from related research is that the negative effects of auditory nerve damage may be overcome by promoting brain plasticity. Intensively training mice as well as humans with or without hearing impairment on sound-in-noise discrimination tasks improved their ability to focus on a target sound (one speaker) amid competing sounds (multiple speakers). This raises the exciting possibility that the maladaptive cortical plasticity and perceptual impairments triggered by an irreversible loss of auditory nerve afferents can be at least partially mitigated by auditory training.
A 2017 Emerging Research Grants (ERG) scientist funded by Hyperacusis Research Ltd., Jennifer Resnik, Ph.D., is a senior lecturer in the department of life sciences at Ben-Gurion University of the Negev in Israel. 2020 ERG scientist Ross Williamson, Ph.D., supported this study with data collection software, and Sharon Kujawa, Ph.D., is a 1999 ERG scientist.






These findings suggest that the ability to integrate what is seen with what is heard becomes increasingly important with age, especially for cochlear implant users.