By Ryan Jaslow, Mass Eye and Ear
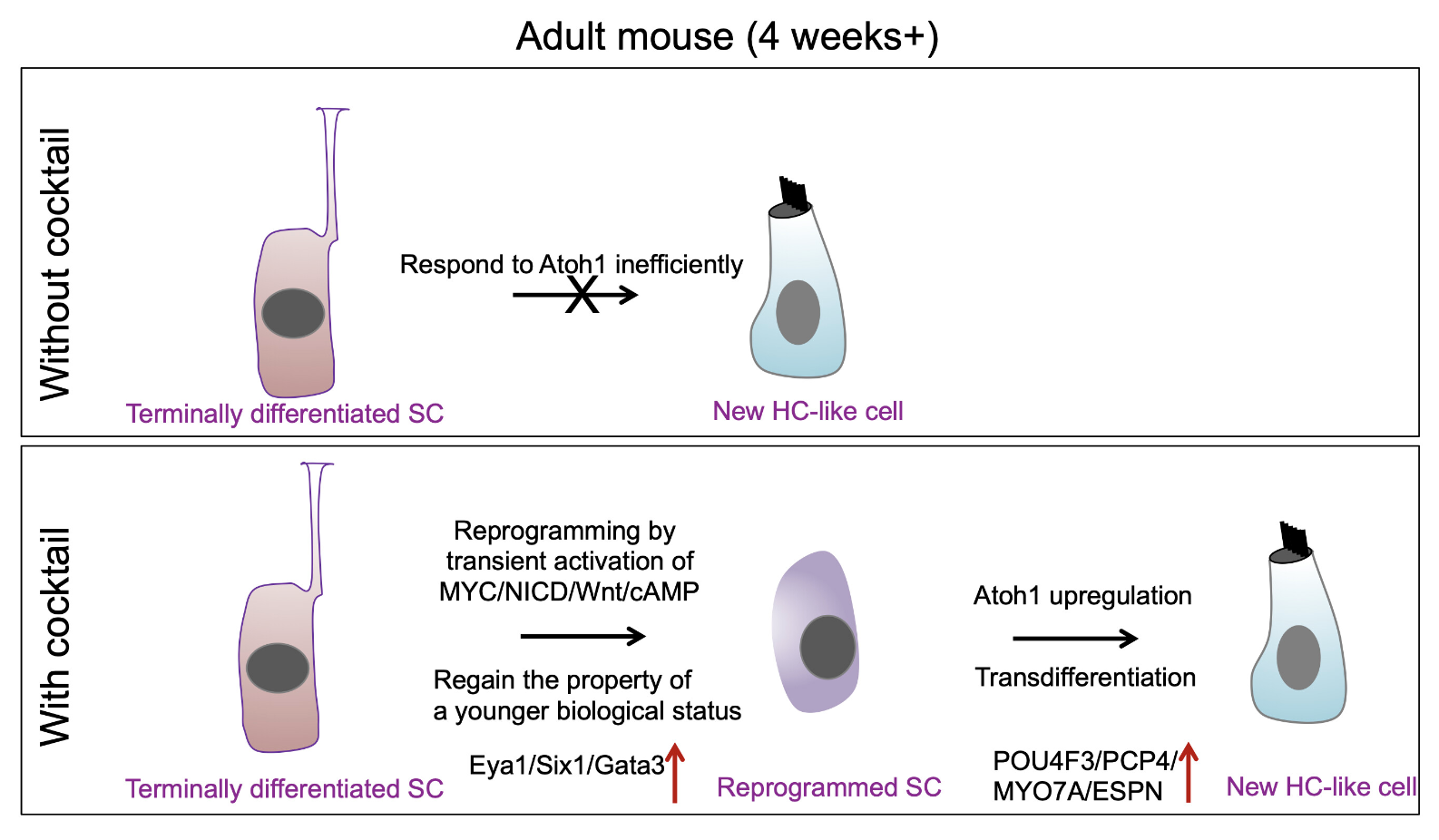
A schematic diagram summarizing the working model for supporting cell (SC) reprogramming by the drug-like molecules and hair cell-like (HC-like) cell regeneration. Credit: Quan et al., PNAS
A research team led by Zheng-Yi Chen, D.Phil., an associate scientist in the Eaton-Peabody Laboratories at Mass Eye and Ear, reports that they have created a drug-like cocktail of different molecules that successfully regenerated hair cell-like cells in a mouse model by reprogramming a series of genetic pathways within the inner ear.
They hope their findings, published in the Proceedings of the National Academy of Sciences (PNAS) in April 2023, could one day pave the way for clinical trials for a gene therapy that can be administered to people with hearing loss.
“These findings are extremely exciting because throughout the history of the hearing loss field, the ability to regenerate hair cells in an inner ear has been the holy grail,” says Chen, who is also an associate professor of otolaryngology–head and neck surgery at Harvard Medical School. “We now have a drug-like cocktail that shows the feasibility of an approach that we can explore for future clinical applications.”
Hearing loss impacts about 48 million Americans and 430 million people worldwide. More than 90 percent of these individuals have sensorineural hearing loss, which is caused by damage to the inner ear and the destruction of hair cells responsible for relaying sounds to the brain.
Hair cells cannot be regenerated in mammals including humans. Unlike other cells in the body, any remaining hair cells in the inner ear cannot divide and other inner ear cells cannot convert themselves into new hair cells. Other species like fish, birds, and reptiles, however, possess this ability.
Previously Chen’s research team studied zebrafish and chickens to uncover which pathways were responsible for inducing the cell division required to regenerate new hair cells. They discovered two molecular signaling pathways, Myc and Notch, were crucial to this process.
In their study published in 2019, they showed for the first time that when these pathways were activated in adult transgenic mice, remaining inner ear cells could divide and develop characteristics of hair cells. The new cells contained transduction channels that relay sound signals and the ability to form connections with auditory neurons—processes essential to hearing.
While an exciting discovery at the time, such an approach was not directly translatable to people, according to Chen. Unlike transgenic mice, humans cannot have Myc and Notch pathways turned on like a light switch. A drug therapy, he says, would have to be introduced to the inner ear to activate the Myc and Notch pathways.
Previous studies have shown that a chemical compound called valproic acid (VPA), can activate Notch, however no molecule exists to effectively activate Myc. That led the researchers to instead look for drug molecules that can alter the downstream pathways that turn on and off when Myc is activated. Through single-cell RNA sequencing, they discovered that activating Myc and Notch led to a downstream effect in which two other pathways, Wnt and cAMP, became activated.
Importantly, they found chemical compounds that can directly activate Wnt and cAMP. They then used small biological molecules called small interfering RNAs (siRNA) to remove genes downstream that suppressed the activation of the Myc pathway.
“Think about a brake when driving a car,” Chen explains. “If the brake is always engaged, you can’t drive. We found an siRNA that could remove the brake in this genetic pathway.”
The researchers then combined the chemical compounds and siRNA molecules into a drug-like cocktail. They delivered it to the inner ear of a normal adult mouse with damaged hair cells—an important distinction, as wild-type, non-transgenic mice are more translatable to humans that genetically modified mice.
They further delivered the gene Atoh1 by a gene therapy approach that utilizes a harmless adenovirus into the cocktail-treated inner ear. Remarkably, they found this drug-like cocktail combined with adenovirus turned on Myc and Notch, which led to the regeneration of new hair cell-like cells. They verified that the hair cells were functional through advanced imaging and other techniques. In the paper’s Discussion, they note the hair cell-like cells’ successes and limitations in more detail.
The researchers are conducting ongoing studies and refinements to this treatment approach in larger animal models, which are necessary before applying to initiate clinical trials. They note that more research is needed to address limitations and challenges for delivering a treatment to the inner ear, and they are examining different gene therapy and surgical methods, including a different viral vector called an adeno-associated virus, which was able to precisely and safely deliver gene therapy to the inner ear through a novel surgery.
“If we can combine a surgical procedure with a refined gene therapy delivery method, we hope we can achieve our number one goal of bringing a new treatment into the clinic,” Chen says.
This is adapted from a Mass Eye and Ear press release. Chen is a 1994 Emerging Research Grants scientist and is a cofounder of Salubritas Therapeutics. In addition to Chen, co-authors of the study are Yizhou Quan, Ph.D., Wei Wei, M.D., Ph.D., Volkan Ergin, Ph.D., Arun Prabhu Rameshbabu, Ph.D., Mingqian Huang, Ph.D., ChunJie Tian, M.D., Ph.D., Srinivas Vinod Saladi, Ph.D., and Artur A. Indzhykulian, M.D, Ph.D.




These findings suggest that the ability to integrate what is seen with what is heard becomes increasingly important with age, especially for cochlear implant users.