By James Dias, Ph.D., and Carolyn McClaskey, Ph.D.
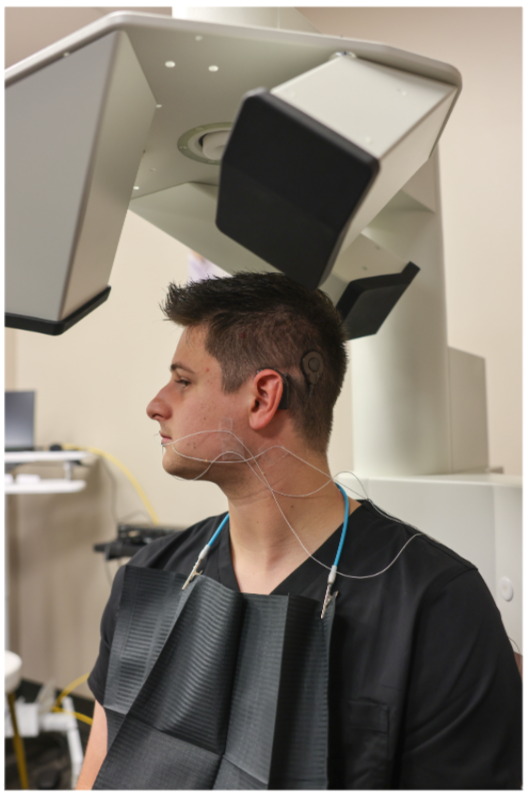
There is a great deal of interest in understanding how the brain can adapt to sensory loss. The structure and function of the brain can change to compensate for a loss in hearing or vision. Unfortunately, many of the techniques used to measure such neuroplasticity can be invasive and expensive. As such, less invasive and more economical techniques to measure and study neuroplasticity can gain a lot of attention.
One such technique, known as tetanization, has been purported to induce neuroplastic changes in the brain by rapidly and repeatedly presenting an auditory or visual stimulus, the tetanus, for a short period of time. Changes in the brain’s response to the tetanus are typically reported as evidence of neuroplasticity.
We recently reviewed the studies employing tetanization to study neuroplasticity in the human brain and conducted a study of our own that tested age-related differences in brain activity following tetanization.
We completed a meta-analysis comparing results across studies, including our own, published in the European Journal of Neuroscience in October 2022. We found that tetanization does not produce reliable changes in brain activity. Some studies report increased brain activity, some report decreased brain activity, and still others report no changes in brain activity following tetanization.
These results are important for developing reliable, noninvasive, and affordable techniques to study hearing loss, vision loss, and neuroplasticity.
James Dias, Ph.D., is an assistant professor in the department of otolaryngology-head and neck surgery at the Medical University of South Carolina. He is a 2022 Emerging Research Grants (ERG) scientist generously funded by the Meringoff Family Foundation, and was renewed for a second year in 2023. One of the study’s coauthors, Carolyn McClaskey, Ph.D., is a research assistant professor in the same department, and is a 2023 ERG scientist generously funded by Royal Arch Research Assistance.








The new public service announcement “Let’s Listen Smart” recognizes that life is loud—and it’s also fun. And the last thing we want to do is stop having fun! We just need to listen responsibly.