The sensory cells in the inner ear and the touch receptors in the skin actually have a lot in common, according to a new study from the University of Southern California (USC) Stem Cell laboratory of Neil Segil, Ph.D., published in the Proceedings of the National Academy of the Sciences on July 20, 2021.
Inner ear sensory cells (left) and skin sensory cells from a one-day-old mouse. Credit: Vincent Yu/Segil Lab/USC Stem Cell
“There are striking similarities in the development of two types of specialized sensory cells: the so-called hair cells that receive sound vibrations in the inner ear, and the Merkel cells that sense light touch at the surface of the skin,” says Segil, who is a professor in the department of stem cell biology and regenerative medicine and in the USC Tina and Rick Caruso department of otolaryngology–head and neck surgery.
A Legacy of Shared Evolutionary History
“Ultimately, these developmental similarities are a legacy of shared evolutionary history,” he says. “This demonstrates how the story of evolutionary developmental biology, or ‘evo devo,’ also extends to what we call the ‘epigenetic level’—or how genes are regulated.”
In the study, doctoral student Haoze (Vincent) Yu, postdoctoral scholar Litao Tao, Ph.D., and colleagues identified a shared mechanism involved in gene regulation, or epigenetics, that enables stem cells and progenitor cells to differentiate into more specialized hair cells and Merkel cells.
In order to begin the process of differentiation, the right parts of a stem cell’s DNA need to be taken out of storage. Each human cell can store around six feet of DNA in its nucleus, because this DNA is wound around tiny “spools” made up of proteins called histones. These spools of DNA and histone protein are further packed together to form what are known are nucleosomes, which are stacked to create chromatin, the material that makes up the chromosomes.
When DNA is wound tightly into this storage configuration, the chromatin is closed and inaccessible to the protein ATOH1. This protein is a “master regulator” that can activate a network of differentiation genes in the DNA within the chromatin—but not without first gaining access.
The Master Regulator and the Pioneer Factor
To this end, ATOH1 stimulates the production of a second protein known as POU4F3, an aptly named “pioneer factor” with the ability to venture into new frontiers by binding to closed and inaccessible chromatin.
After POU4F3 blazes a trail by binding to the closed chromatin, ATOH1 is able to move forward with engaging and activating the network of genes that drives differentiation into hair cells and Merkel cells.
Strikingly, there is significant overlap in the specific regions of chromatin that POU4F3 makes accessible to ATOH1 in hair cells and Merkel cells.
An Ancient Mechanism in Hearing
“It’s remarkable that these two cell types, which are both involved in sensing mechanical stimuli but derive from distinct parts of the embryo, both rely on the same ATOH1/POU4F3 mechanism in order to differentiate,” Segil says. “Our study suggests that this mechanism is extremely ancient, and emerged before hair cells and Merkel cells diverged from a common evolutionary ancestor—an ‘ur-mechanoreceptor’ cell type.”
This is adapted from a USC press release. Neil Segil, Ph.D., a member of Hearing Health Foundation’s Hearing Restoration Project, is a professor in the University of Southern California’s department of stem cell biology and regenerative medicine and the USC Tina and Rick Caruso department of otolaryngology–head and neck surgery.



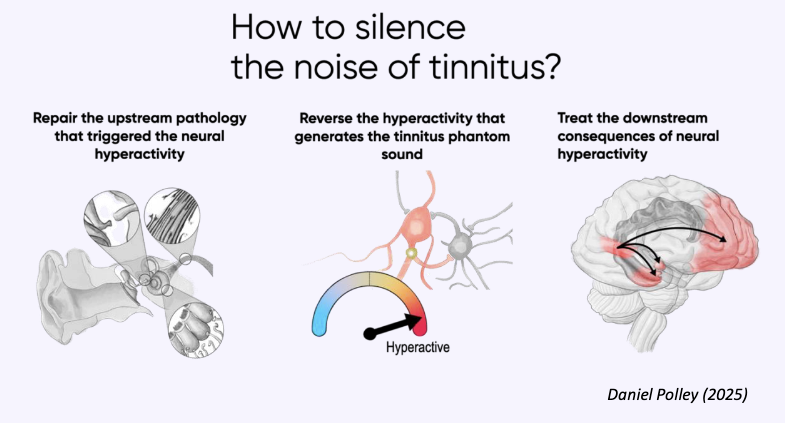
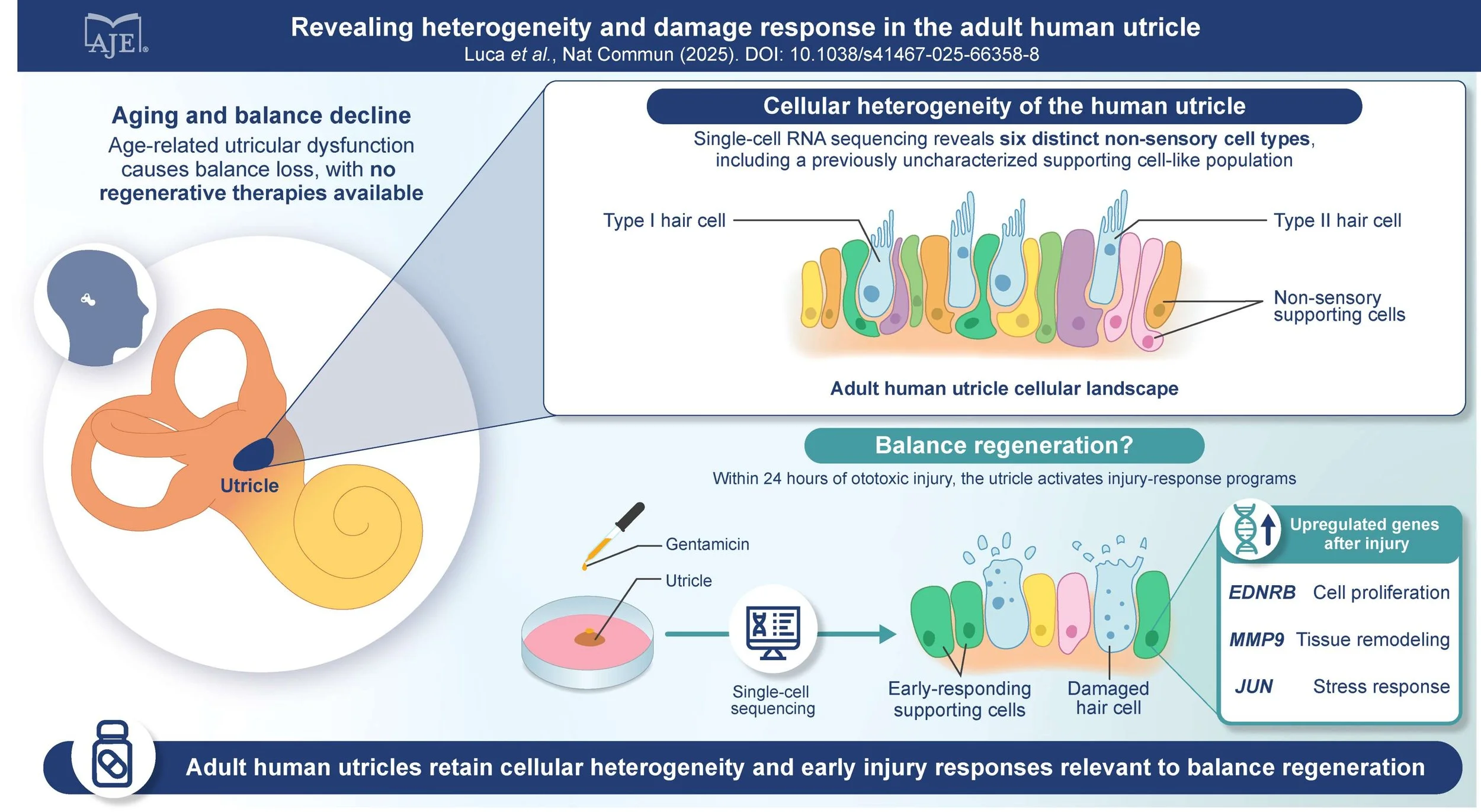

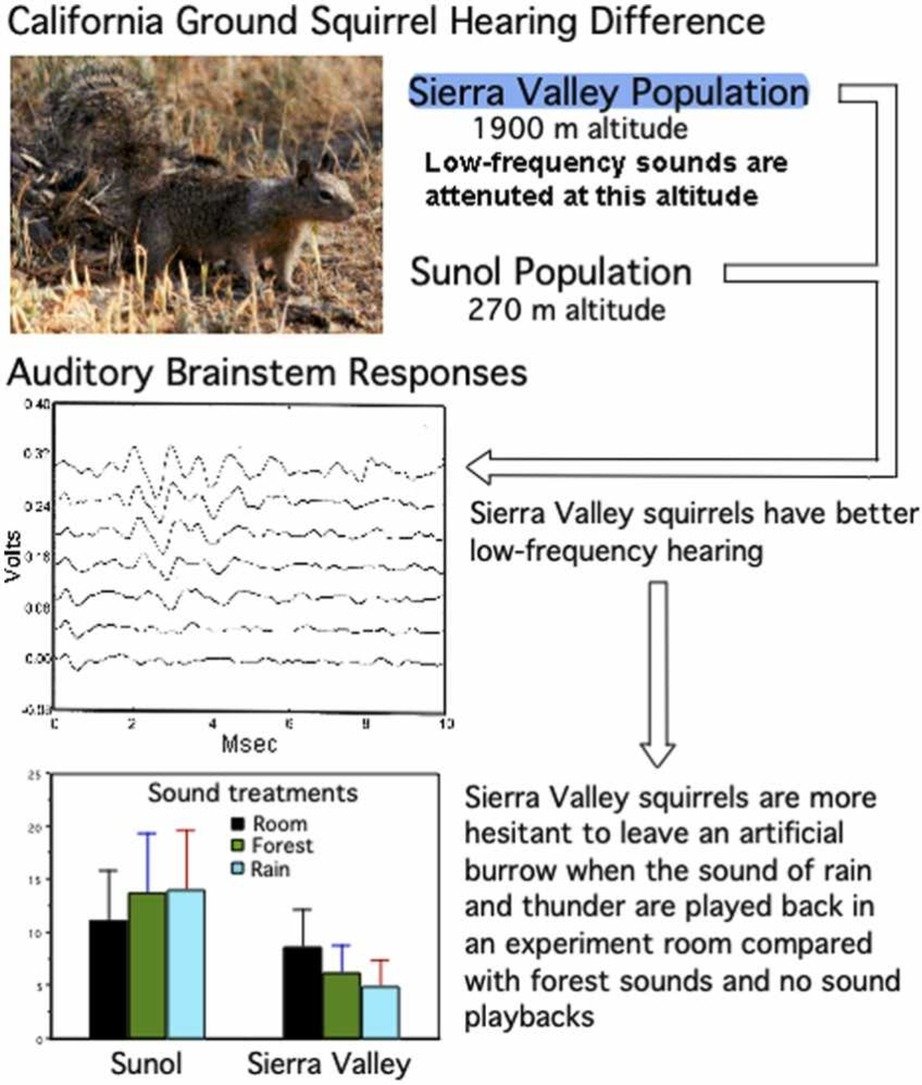

These findings suggest that the ability to integrate what is seen with what is heard becomes increasingly important with age, especially for cochlear implant users.