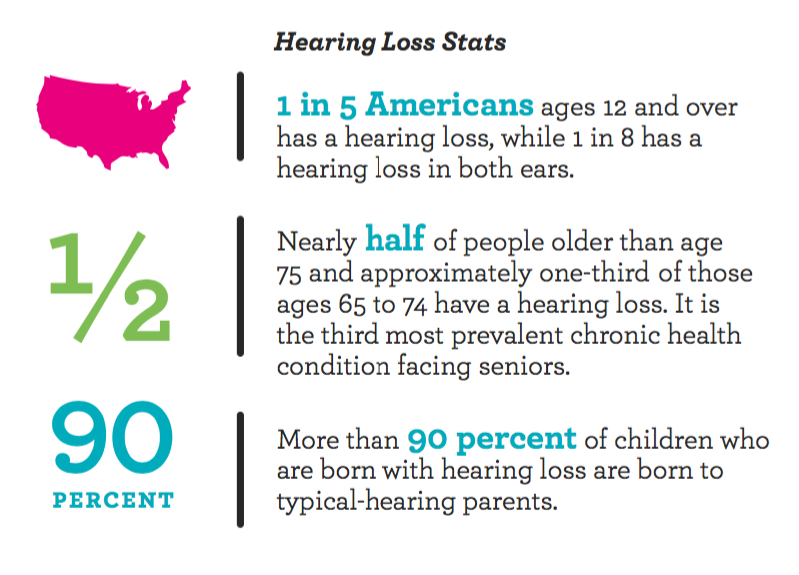
Hearing regeneration is already possible in frogs, fish, and yes, chickens. With your help, the members of HHF’s Hearing Restoration Project (HRP) can accelerate their studies to identify permanent cures and better the lives of millions.
HHF Achieves Accreditation From BBB Wise Giving Alliance
By Morgan Leppla
Accountable, transparent, responsive, and enterprising.
It takes a resolute organization to embody these traits. While we believe that has been the case since Hearing Health Foundation’s inception in 1958, we now have Better Business Bureau Wise Giving Alliance (BBB WGA) accreditation to prove it!
The BBB WGA evaluates charities based off of 20 holistic standards that include scrutinizing financial transparency and planning, internal governance, effectiveness measurements, and fundraising disclosure practices and accuracy. Check out our profile at Give.org today!
We also recently received a Platinum rating from Guidestar, which is the highest rating available. This rating signifies that HHF yields measurable results using self-defined metrics that reveal much more than oversimplified financial ratios.
But why should this matter to you?
Because these 3rd party ratings show we achieve our mission, responsibly!
“The public can be assured that every charity evaluation is completed with careful, objective analysis of charity information,” says Art Taylor, president and CEO of the BBB Wise Giving Alliance. “
The more we become deserving of your trust, the better the prospects for curing and preventing hearing loss and tinnitus. More than 80 cents of every dollar goes to funding programs and research, meaning we have the capacity to to enact the changes we promise.
While the BBB seal of approval verifies the standards of our operating procedures described above, our commitment to quality is motivated by a much greater force. It is traceable to our mission and core values, which structure the ways we act and choices we make. Accountability to our constituents and stakeholders is crucial to upstanding practices, and without it we would not be HHF.
Please consider making a gift today so we can continue to carry out our mission and find a cure for hearing loss and tinnitus.
Unlocking the Potential for Hair Cell Regeneration
By Laura Friedman
On November 5, 2015, Hearing Health Foundation hosted its second live-video research briefing as part of our effort to provide regular updates on our research programs and progress. Through these briefings, our goal is for our attendees to obtain new information and understanding about hearing loss, prevention and research toward a cure.
Dr. Andy Groves, Hearing Restoration Project consortium member, presented recent research advances and new discoveries, the use of new technology, and our future plans to prevent and cure hearing loss and tinnitus. The HRP was founded in 2011 and is the first and only international research consortium focused on investigating hair cell regeneration as a cure for hearing loss and tinnitus. The overarching principle of the consortium is collaboration: open sharing of data and ideas. The HRP consortium consists of 13 of the top investigators in the audiological space, as well as a scientific director, Dr. Barr-Gillespie.
We wanted to share with you highlights from the presentation, which is available to watch with live captioning or to read with notes summarizing each slide.
Your Support Is Needed!
Hair cell regeneration is a plausible goal for eventual treatment of hearing and balance disorders.
The question is not if we will regenerate hair cells in humans, but when.
However, we need your support to continue this vital research and find a cure! Please make your gift today.
#HearTheHope This Holiday Season
By Laura Friedman
#GivingTuesday 2015, an international day of giving that kicks off the holiday giving season, is just around the corner on December 1st.
Hearing Health Foundation (HHF) wants to thank you for your continued support of our mission and programs, such as the Hearing Restoration Project (HRP) and Emerging Research Grants (ERG). Your support matters and has, and will continue to, enhance the lives of millions of Americans. Here are some of our successes, dating back to our founding in 1958:
HHF is the largest private funder of hearing research in the U.S.
HHF funded research has led to:
The development of cochlear implants
Treatments for otosclerosis (abnormal bone growth in the ear) and ear infections.
In 1985, scientists funded through the ERG program discovered that chickens regenerate their inner ear hair cells after damage and mammals do not. This study led to the development of the HRP in 2011.
In the 1990s HHF advocated for Universal Newborn Hearing Screening legislation, to detect hearing loss at birth.
Today, 97% of newborns are tested (up from 4% in 1994).
The work doesn't stop there. Your support will continue to impact the course of hearing and balance science and help us find a cure for the 50 million Americans living with hearing loss and tinnitus. The question of finding a cure for hearing loss is not if, but when. Making a financial commitment to HHF is an investment in our future. But we need YOUR help. Here are some ways you can #HearTheHope this holiday season:
Make a donation to HHF in honor or in memory of someone close to you.
Post on social media, such as Facebook or Twitter, encouraging your friends to donate to HHF.
The average person has 300 friends on Facebook which means that if each of your friends donates just $1 on Giving Tuesday, you can raise $300 in one day—it’s that easy!
Contribute to an item on our Wish List and give our researchers the tools they need.
You can make gifts of appreciated stocks or a planned gift!
Let your talents and interests lead you to your own fundraiser for HHF through our website! No event is too large or small. Here are some ideas for inspiration:
Host a potluck and invite your guest to join you by bringing a dish and making a donation to HHF.
Hold a bake sale or golf outing and advertise that the proceeds will be donated to HHF.
Burn excess Thanksgiving calories and go for a run, swim (indoors of course!), or bike ride, fundraising for every mile accomplished.
Have other ideas or questions for us? E-mail us at Development@hhf.org.
Any donation you send before December 31st will be instantly doubled thanks to a generous matching gift from one of our supporters with hearing loss — and you will make twice the IMPACT!
How Hearing Loss and Tinnitus Affect Our Veterans
By Emily Shepard
Today is Veterans Day. The holiday is important not only because it honors our soldiers, but also because it is a time to raise awareness about their experiences on and off the battlefield. Hearing loss is a major health issue for soldiers, both active duty personnel and veterans. Any form of hearing loss can be detrimental to soldiers on duty, as the ability to hear signs of danger and to communicate with fellow soldiers is crucial for mission success and, more importantly, survival. According to the U.S. Department of Defense’s Hearing Center of Excellence (HCE), a whopping 60% of veterans have returned home with hearing loss or tinnitus over the last decade.
The Fall 2015 issue of Hearing Health magazine focused on hearing loss and tinnitus among U.S. military service members and veterans. In “Tuning Out the Noise,” Ashleigh Byrnes explains that tinnitus is one of the most prevalent injuries among veterans. The number of veterans diagnosed with service-connected tinnitus is estimated at 1.5 million. According to Byrnes, persistent tinnitus can be “described as noise that prevents sleep or the ability to concentrate” and may “leave patients more vulnerable to other mental health problems, such as depression and anxiety.” Luckily, there are treatment methods, new and old, that can ease the symptoms of tinnitus.
Sound therapy, long regarded as one of the most successful ways to treat tinnitus, has been practiced for more than 30 years. Between 60-90% of patients report relief from their symptoms using this method. Another option is cognitive behavioral therapy (CBT), which may include the use of relaxation or distraction techniques, or altering the way patients think about their symptoms. Those who try sound therapy or CBT may be able to cope with tinnitus with more positive outcomes.
When it comes to hearing loss, soldiers are at an increased risk. They are susceptible to noise-induced hearing loss (NIHL) due to exposure to loud machinery and explosions on a constant basis. In combat, soldiers are often exposed to sudden noises, such as from an improvised explosive device (IED) or other similar weapons, which are difficult to predict and prevent against. These sudden noises can result in temporary hearing loss and put military personnel at risk. However, the word “temporary” should be approached with caution. Repeated short-term hearing loss can damage the sensitive hair cells in the inner ear, causing hearing loss that becomes permanent.
With an inability to grow back, inner ear hair cells, when they are damaged or die, can lead to permanent hearing loss. HHF is actively working to reverse this trend. Researchers funded by HHF’s Emerging Research Grants program (ERG) discovered that birds have the ability to spontaneously re-grow inner ear hair cells after they are damaged and restore their hearing—unlike mammals. Through HHF’s Hearing Restoration Project (HRP), a consortium of top hearing scientists is working to translate this finding to the human ear. The HRP’s goal is to regenerate inner ear hair cells in humans and permanently restore hearing to those affected by hearing loss, such as soldiers and veterans. The HRP researchers have made significant strides in this research and have been working hard to find meaningful answers, which you can read about here.
To learn more about hearing loss and tinnitus, please visit our Veterans’ Resource Page.
Your support helps us continue this extraordinary research.
Celebrate Veterans Day and honor our troops by donating today.
Spotlight On: Andy Groves, Ph.D.
CURRENT INSTITUTION:
Baylor College of Medicine, Houston, Texas
EDUCATION:
Undergraduate from the University of Cambridge
Ph.D. from the Ludwig Institute for Cancer Research, London
Postdoc at the California Institute of Technology
This new feature aims to connect Hearing Health Foundation (HHF) supporters and constituents to its Hearing Restoration Project (HRP) consortium researchers. Spotlight On provides an opportunity to get to know the life and work of the leading researchers working collaboratively in pursuit of a cure for hearing loss and tinnitus.
What is your area of focus?
I am a developmental biologist who uses the ear as a model system to understand the general problem of embryonic development—how do you form something very complicated from very simple beginnings. The inner ear is a tissue that receives extremely precise instructions to form just the right number of cells in the right place at the right time. My lab studies where the ear comes from embryonically, how the cochlea acquires its exquisite pattern, and why sensory hair cells are not replaced in mammals after damage.
Why did you decide to get in to scientific research?
I always enjoyed biology and chemistry as a kid and thought it would be more fun than studying medicine. I had a very enthusiastic high school biology teacher who loaned me books on biology and evolution, which made an enormous impression. When I was an undergraduate at Cambridge, I was lucky to have two professors who both won Nobel Prizes, and during my senior year I had the opportunity to do research with one of them. After that, scientific research seemed like the only game in town….
Why hearing research?
I started to study ear development as a postdoctoral fellow in the 1990s because it had received very little attention for decades. The ear appeals to my love of extremes in biology: It has one of the most elaborate three-dimensional structures of any organ; it possesses cells of astonishing mechanical sensitivity; and it can detect sounds over a trillion-fold power range. It is also remarkable to think that our entire auditory experience—conversation, music, the natural world—is captured by just a few thousand sensory cells in each ear!
What is the most exciting part of your research?
Experiments can take months or years to carry out. But every now and then you find something new, and the thrill of realizing that you have found out something that no one else in the world knows about is quite addictive.
What do you enjoy doing when you’re not in the lab?
I am a huge music fan and have a large CD collection. Right now my playlist includes Beethoven sonatas played on a fortepiano, some rare Miles Davis live concerts from 1965, and Howlin’ Wolf albums. As a grad student, I sang at Cambridge and with the London Philharmonic Orchestra. I also love reading. Despite living in the U.S. for over two decades, I know very little about its history, so I have been trying to educate myself about the Civil War Era. I just finished reading “The Half Has Never Been Told” by Edward Baptist.
What is a memorable moment from your career?
For me, it is the “firsts”—seeing students or postdocs publish their first paper or when someone in my lab gets their first academic position. The nature of science means that most of what is discovered will become obsolete or surpassed, but the achievements and careers of the people who have come through the lab will hopefully last for much longer.
If you weren’t a scientist, what would you have done?
To be honest, I never had a “plan B.” I love teaching, and so if I had to give up research, it might be nice to teach biology to undergraduates.
Hearing Restoration Project
What has been a highlight from the HRP consortium collaboration?
The biggest help has been having collaborators on hand to do experiments that are outside the scope of my own lab. We recently published a paper with another HRP researcher, Stefan Heller, Ph.D., at Stanford, where he helped us analyze gene expression of single cells in the cochlea. We showed that blocking the Notch pathway could cause new hair cells to form in very young animals, but that this approach stops working as animals get older. The explosion of new technology and techniques means it is harder to do all the experiments you want in your own lab—so collaboration is key.
What do you hope to have happen with the HRP over the next year, two years, five years?
I hope we can begin a large-scale testing of candidate drugs or gene manipulations in the next two years. This initial screening will likely be in cell culture systems or in the zebrafish system that some members of the HRP helped to pioneer. In five years, I hope we have lead compounds that have been validated independently in several HRP labs.
What is needed to help make HRP goals happen?
Frankly, funding to keep our research moving forward. A postdoctoral fellow with five to six years of training starts out on a modest salary of about $45,000, plus $12,000 in benefits. So that’s $57,000 before they even pick up a test tube in the lab. Each person will typically use between $15,000-$20,000 a year in supplies and chemicals. Simply maintaining a single cage of mice for one year costs $210, and my lab can use between 300-500 cages of mice for our experiments! HHF and its donors have been extremely generous in their support, however with additional funding the output from the consortium could be significantly greater and accelerate the pace to a cure.
Which scientist or mentor was the most inspirational?
My two postdoctoral mentors at Caltech, David Anderson and Marianne Bronner, were both instrumental in making me the scientist I am today. As I was moving into the ear field, I was also lucky to meet Ed Rubel while he was on a sabbatical at Caltech and now as a fellow member of the HRP. More broadly, my two scientific heroes are Seymour Benzer and Francis Crick. Both were gifted scientists who laid the foundations of modern biology and were able to make seminal contributions to every field they worked in, from developmental and molecular biology to the study of aging, behavior, and consciousness.
Your financial support will help ensure we can continue this vital research in order to find a cure for hearing loss and tinnitus in our lifetime. Please donate today to fund the top scientific minds working collaboratively toward a common goal. For more information or to make a donation, email us at info@hhf.org.
Your help provides hope.
Distilling the Data
By Michael Lovett, Ph.D.
The burgeoning field of bioinformatics allows the Hearing Restoration Project to analyze and compare large genomics datasets and identify the best genes for more testing. This sophisticated data analysis will help speed the way toward a cure for hearing loss and tinnitus.
Since its launch in 2011, the Hearing Restoration Project (HRP) is focused on identifying new therapies that will restore inner ear hair cell function, and hence hearing. Within the consortium, smaller research groups engage in separate projects over the course of the year, to move the science along more quickly.
Over the past decade my group, and the group led by my collaborator Mark Warchol, Ph.D., have worked to identify genes that are potential targets for drug development or for gene therapies to cure hearing loss. Our approach has been to determine the exact mechanisms that some vertebrates—in our case, birds—use to regenerate their hair cells and thus spontaneously restore their hearing. We have been comparing this genetic “tool kit” with the mechanisms that mammals normally use to make hair cells.
Unlike birds, mammals cannot regenerate adult hair cells when they are damaged, which is a leading cause of human hearing and balance disorders. Our working hypothesis is that birds have regeneration mechanisms that mammals are missing—or that mammals have developed a repressive mechanism that prevents hair cell regeneration.
In either case, our strategy has been to get a detailed picture of what transpires during hair cell regeneration in birds by using cutting-edge technologies developed during the Human Genome Project (the international research collaboration whose goal was the complete mapping of all the nuclear DNA in humans). These next-generation (NextGen) DNA sequencing methods have allowed us to accurately measure changes in every single gene as chick sensory hair cells regenerate.
The good news is that this gives us, for the first time, an exquisitely detailed and accurate description of all of the genes that are potential players in the process. The bad news is that this is an enormous amount of information; thousands of genes change over the course of seven days of regeneration.
Some of these will be the crucially important—and possibly game-changing—genes that we want to explore in potential therapies, but most will be downstream effects of those upstream formative events. The challenge is to correctly identify the important causative needles in the haystack of later consequences.
We already know some important genetic players, but we are still far from understanding the genetic wiring of hair cell development or regeneration. For example, after decades of basic research, we know that certain signaling pathways, such as those termed Notch and Wnt, are important in specifying how hair cells develop. These chemical signaling pathways are made of multiple protein molecules, each of which is encoded by a single gene.
However, the Notch and Wnt pathways together comprise fewer than 100 genes and, despite being intensively studied for years, we do not completely understand every nuance of how they fit together.
It also may seem surprising that—more than a decade after the completion of the Human Genome Project and projects sequencing mouse, chick, and many other species’ genomic DNA—we still do not know the exact functions of many of the roughly 20,000 genes, mostly shared, that are found in each organism. This is partly because teasing out all of their interactions and biochemical properties is a painstaking process, and some of the genes exert subtly different effects in different organs. It is also because the genetic wiring diagram in different cells is a lot more complicated than a simple set of “on/off” switches.
All of this sounds a bit dire. Fortunately, we do have some tools for filtering the data deluge into groups of genes that are more likely to be top candidates. The first is to extract all of the information on “known” pathways, such as the Notch and Wnt mentioned earlier. That is relatively trivial and can be accomplished by someone reasonably well versed in Microsoft Excel.
That leaves us with the vast “unknown” world. Analyzing this requires computational, mathematical, and statistical methods that are collectively called bioinformatics. This burgeoning field has been in existence for a couple of decades and covers the computational analysis of very large datasets in all its forms. For example, we routinely use well-established bioinformatic methods to assemble and identify all of the gene sequences from our NextGen DNA sequence reads. These tasks would take many years if done by hand, but a matter of hours by computational methods.
In the case of our hair cell regeneration data, our major bioinformatic task is to identify the best genes for further experimental testing. One method is to computationally search the vast biological literature to see if any of them can be connected into new networks or pathways. There are now numerous software tools for conducting these types of searches. However, this really is not very helpful when searching through several thousand genes at once. The data must be filtered another way to be more useful.
We have used statistical pattern matching tools called self-organizing maps to analyze all of our data across every time point of hair cell regeneration. In this way we can detect genes that show similar patterns of changes and then drill down deeper into whether these genes are connected. This has provided us with an interesting “hit list” of genes that have strong supporting evidence of being good candidates for follow-up.
An additional approach is to compare our chick data to other datasets that the HRP consortium is collecting. The logic here is that we expect key genetic components to be shared across species. For example, we now know a great deal about what genes are used in zebrafish hair cell regeneration and the genes that specify mouse hair cells during normal development. We can conduct computational comparisons across these big datasets to identify what is similar and what is different. Again, this has yielded a small and interesting collection of genes that is being experimentally tested.
Our final strategy has been to extract classes of genes that act as important switches in development. These transcription factors control other genetic circuits. We have identified all of these that change during chick hair cell regeneration. As a consortium the HRP now has a collection of about 200 very good candidate genes for follow-up. However, software and high-speed computation are not going to do it all for us. We still need biologists to ask and answer the important questions and to direct the correct bioinformatics comparisons.
Hair cell regeneration is a plausible goal for the treatment of hearing and balance disorders. The question is not if we will regenerate hair cells in humans, but when. Your financial support will help to ensure we can continue this vital research and find a cure in our lifetime! Please help us accelerate the pace of hearing and balance research and donate today. Your HELP is OUR hope!
If you have any questions about this research or our progress toward a cure for hearing loss and tinnitus, please contact Hearing Health Foundation at info@hhf.org.
Michael Lovett, Ph.D., is a professor at the National Lung & Heart Institute in London and the chair in systems biology at Imperial College London.
Ask the Scientist: Gene Therapies and Hearing
By Peter G. Barr-Gillespie, Ph.D.
A DNA double helix
National Human Genome Research Institute
Recently, Hearing Health Foundation (HHF) has received several questions regarding the Reuters report on gene therapies for hearing. There are two separate but related topics raised in this article. As the scientific research director of HHF’s Hearing Restoration Project, which since 2011 has been uncovering concrete discoveries toward a biologic cure for hearing loss and tinnitus, I want talk about each individually, and then discuss what I interpret they mean together.
The article first presents the Science Translational Medicine paper from Jeffrey Holt’s lab. This is very much a proof-of-principle report, focused on an animal model and using a time for delivery of the corrected gene that is extremely early in development (equivalent to a 5-to-6-month-old human fetus). It is important to point out that their strategy will only correct one type of genetic hearing loss and genetic hearing loss from mutations in other genes will require related but different strategies. Nevertheless, this is an exciting example of modeling gene therapy in animals, and represents a logical progression toward that goal in humans.
The article then moves on to reference the Novartis trial. For this trial, they are using a similar technical strategy, viral delivery of a gene, but they are targeting people—those who have lost their hearing through non-genetic means, such as noise damage, aging, or infections. The gene they are delivering, known as ATOH1, may stimulate production of new hair cells; it is a gene that is essential for formation of hair cells during development, and in some experimental animal models, delivery of the gene can lead to production of a few hair cells in adult ears.
That said, many people who I have talked to in the field who work with experimental models of hair cell formation using ATOH1, including members of our Hearing Restoration Project consortium, believe that this trial is premature. By and large, the animal models do not support the trial; most suggest that there will be few hair cells formed and little hearing restored. While we can hope for a little bit of hearing recovery, we are concerned about toxic responses to the gene delivery using viruses. Personally, while I think it would be truly fantastic if the Novartis trial works, at this moment in time I don’t think the rewards yet outweigh the considerable risks being imposed on a human (include safety during the procedure and potential side effects afterward).
Still, the Novartis trial will tell us about the safety of viral delivery into the ears of humans, and knowing that is critically important. I think the most likely outcome is that we will learn whether the strategy the Novartis trial used to deliver the gene is safe. Unfortunately, if we don’t see improved hearing, we won’t know why—did the gene not get to the right place, or does it just not work?
Technical aspects of gene delivery are what ties together the Novartis work and the Holt lab work. Both use viruses for delivering genes, and together the results from these and others will let us know, from a procedural standpoint, how we can deliver genes to the ear. I think it is unlikely that delivering just ATOH1 will do the trick of restoring hearing; it may be that we need to deliver other genes or to use drugs to overcome the block we see to making new hair cells.
So while these are exciting reports to hear about, especially that Novartis is actually carrying out a trial in humans, it is still premature to think that this is going to be a viable strategy for restoring hearing. This is why Hearing Health Foundation's Hearing Restoration Project is doing everything possible to accelerate the pace of its research.
Hair cell regeneration is a plausible goal for the treatment of hearing and balance disorders. The question is not if we will regenerate hair cells in humans, but when. Your financial support will help to ensure we can continue this vital research and find a cure in our lifetime! Please help us accelerate the pace of hearing and balance research and donate today. Your HELP is OUR hope!
If you have any questions about this research or our progress toward a cure for hearing loss and tinnitus, please contact Hearing Health Foundation at info@hhf.org.
The Path to a Cure for Hearing Loss and Tinnitus
By Laura Friedman
On May 21, 2015, Hearing Health Foundation hosted its first live-video research briefing as part of our effort to provide regular updates on our research programs and progress. Through these briefings, our goal is for our attendees to obtain new information and understanding about hearing loss, prevention and research toward a cure.
During this inaugural research briefing, Dr. Peter Barr-Gillespie, Scientific Director, Hearing Restoration Project presented the Hearing Restoration Project (HRP). The HRP was founded in 2011 and is the first and only international research consortium focused on investigating hair cell regeneration as a cure for hearing loss and tinnitus. The overarching principle of the consortium is collaboration: open sharing of data and ideas. The HRP consortium consists of 14 of the top investigators in the audiological space, as well as a scientific director, Dr. Barr-Gillespie.
We wanted to share with you highlights from the presentation, which is available to watch with live captioning or to read with notes summarizing each slide.
History of Hearing Health Foundation
Founded in 1958, established reputation for pioneering breakthroughs in hearing and balance research.
Early supporters of the revolutionary cochlear implant. Today, over 220,000 children and adults benefit.
Advocated for the passage of Universal Newborn Hearing Screening legislation in the 1990s. Today, 97% of newborns are tested for hearing loss at birth.
The Emerging Research Grants Program provides seed funding for researchers in hearing and balance science such as discoveries in hair cell regeneration, tinnitus, hyperacusis, and Ménière’s research.
The Challenge
In the past century, the primary treatment for hearing loss has been hearing aids and cochlear implants. While these have been very successful treatments, they have limitations.
For this century, we have a number of different avenues for more effective therapy.
Preventing the damage to the hair cells to preserve hearing. By generating greater awareness of the effects of hearing loss, we aim encourage people of all ages to protect their ears.
Gene therapy, targeting those who have lost hearing due to genetic disorders.
The majority of people who have lost hearing have done so through noise damage or aging, and may be candidates for hair cell regeneration/restoration.
HRP Consortium History & Model
One of the key facets of the HRP’s approach is that we use three different animal models for studying hair cell regeneration
Two of those models, the chick and the zebrafish, show robust hair cell regeneration.
f you damage the hair cells of a chick or a fish, within a short time—only a day or two for the fish, a few weeks for the chick—the hair cells come back; new hair cells are formed.
So, that's spectacular, because it tells us that animals are capable of regenerating hair cells.
y contrast, the mouse is our other experimental model. Like in the human, the mouse shows no hair cell regeneration after a few days following birth.
You can damage the hair cells in the mouse and as far as we can tell, nothing much happens in terms of restoring hair cells. So, if we can figure out how to regenerate hair cells in the mouse, then we will be able to regenerate hair cells in people.
HRP Strategic Research Plan
Our strategic plan consists of three separate phases. We have already made a lot of progress on Phase 1 and we have initiated Phase 2:
Phase 1 – Discovery research: Compare the fish, chick, and mouse to discover pro- or anti-regeneration pathways and determine supporting cell fates.
Phase 2 – Pathway validation: Verify pathways using fish, chick, and mouse models and describe regeneration strategies.
Phase 3 – Develop therapies and treatment options: Identify drugs that trigger hair cell regeneration in the mouse model.
Progress To-Date
Progress on Phase 1: We've identified a variety of candidates for hair cell regeneration and the pathways that are necessary.
We have too many, so we really are continuing to use bioinformatics methods to winnow down and determine which are most important.
We have definitively shown, at least in the mouse, the specialized supporting cells remain.
We know now what our target cells are for triggering hair cell regeneration.
Phase 2 has begun, but we haven’t stopped Phase 1:
We've got multiple approaches to try and see whether or not we can block regeneration in the fish and chick or stimulate regeneration in the mouse.
Phase 3 is in sight:
Experimental models from Phase 2 will be used to screen for drugs—using the mouse first
The Next Five Years
With your help, we can continue to quicken the pace towards a cure. Here’s our plan for the next five years:
Phase 1 will continue: more candidate generation for Phase 2
Phase 2 (pathway verification) already initiated in zebrafish, mouse, chick (low throughput)
Phase 2 must be scaled up: many more genes, combinatorial approaches; cell lines for screening
Phase 3 (drug screening) requires the right screening model, which will come out of Phase 2.
The Future is Very Bright – But we need your support!
Hair cell regeneration is a plausible goal for eventual treatment of hearing and balance disorders. The question is not if we will regenerate hair cells in humans, but when. However, we need your support to continue this vital research and find a cure! Please make your gift today.
Hearing Makes Me Happy!
By Alex Mussomeli
By all accounts Alex Mussomeli is a typical elementary school kid; he likes art, music, sports, cooking, and video games. But what is phenomenal about Alex is how much he is comfortable with and unfazed by his hearing loss, and also how much, for a 10-year-old, he understands the technology that helps him to hear. Diagnosed with sensorineural hearing loss, Alex was fitted with hearing aids in both ears at age 3 months, and when he was 3 years old, he got a cochlear implant for his right ear. He continues to use both devices.
When his fourth grade teacher asked her students to write on a topic they know a lot about, Alex chose hearing loss and his hearing devices. The paper impressed the hearing-speech pathologist at school so much that it was shared on Speech4Hearing.com, a website that offers speech advice for parents of children with hearing loss.
It also impressed us at Hearing Health Foundation (HHF). Here are some excerpts:
“There are two ways to help people with hearing loss hear,” Alex wrote. “One is a common way, a hearing aid, and the other way is not as common but is getting more common every day, which is a cochlear implant. I have both.”
“The reason people might need to get a cochlear implant is that they might not hear. They could be deaf or have hearing loss. The surgery of getting an implant can be a big decision. First the nurses give the patient sleeping medicine. Then, the surgeon drills into the skull. Next, the surgeon puts in a magnet. After the surgery you have to wait one month for the head to heal from the surgery.”
Once the healing period is over, the implant is turned on and it is programmed, or mapped, to fit the specific hearing requirements of the patient, and then the brain has to learn to process the sounds that the implant picks up and delivers directly to the brain via the auditory nerve. Nada Alsaigh, Alex’s mother, says Alex’s young age worked in his favor. “We were very lucky,” she says. “He was very fast learning how to use the implant to hear.”
“The way you hear with a hearing aid is like a first aid kit. The hearing aid assists the person in hearing,” Alex wrote in his paper. “An implant is better than a hearing aid because you can hear better with it. The reason is that the implant has a computer like processor that sends the sound through the nerve to the brain.”
Alex and his brother Joe, who is 12, are incredibly close. “We face challenges and we try to overcome them. This was a learning experience for all of us and made Joe more mature at a younger age. Joe is just a loving and supportive brother,” Nada Alsaigh says of Joe. The family treats Alex’s hearing loss as a part of who he is without defining who he is.
While thankful for existing technology, the family is also committed to helping HHF and its Hearing Restoration Project (HRP) find a cure for hearing loss and tinnitus. The HRP is on track to determine how to regenerate the inner ear sensory cells that, when missing or damaged, lead to hearing loss. The promise of a biologic cure for hearing loss could potentially remove the issues of adapting to hardware for Alex and the 50 million other adults and children in the U.S. who have hearing loss.
Smart and thriving, Alex realizes he is fortunate:
"Hearing makes me happy!” Alex says. “I am grateful that my parents got me a hearing aid and a cochlear implant. Someday, I believe that we will find a cure to have hearing cells come back to life! We can't give up hope! Hearing Health Foundation can help us find the cure!"
Alex's paper on hearing loss is availible in full, here.