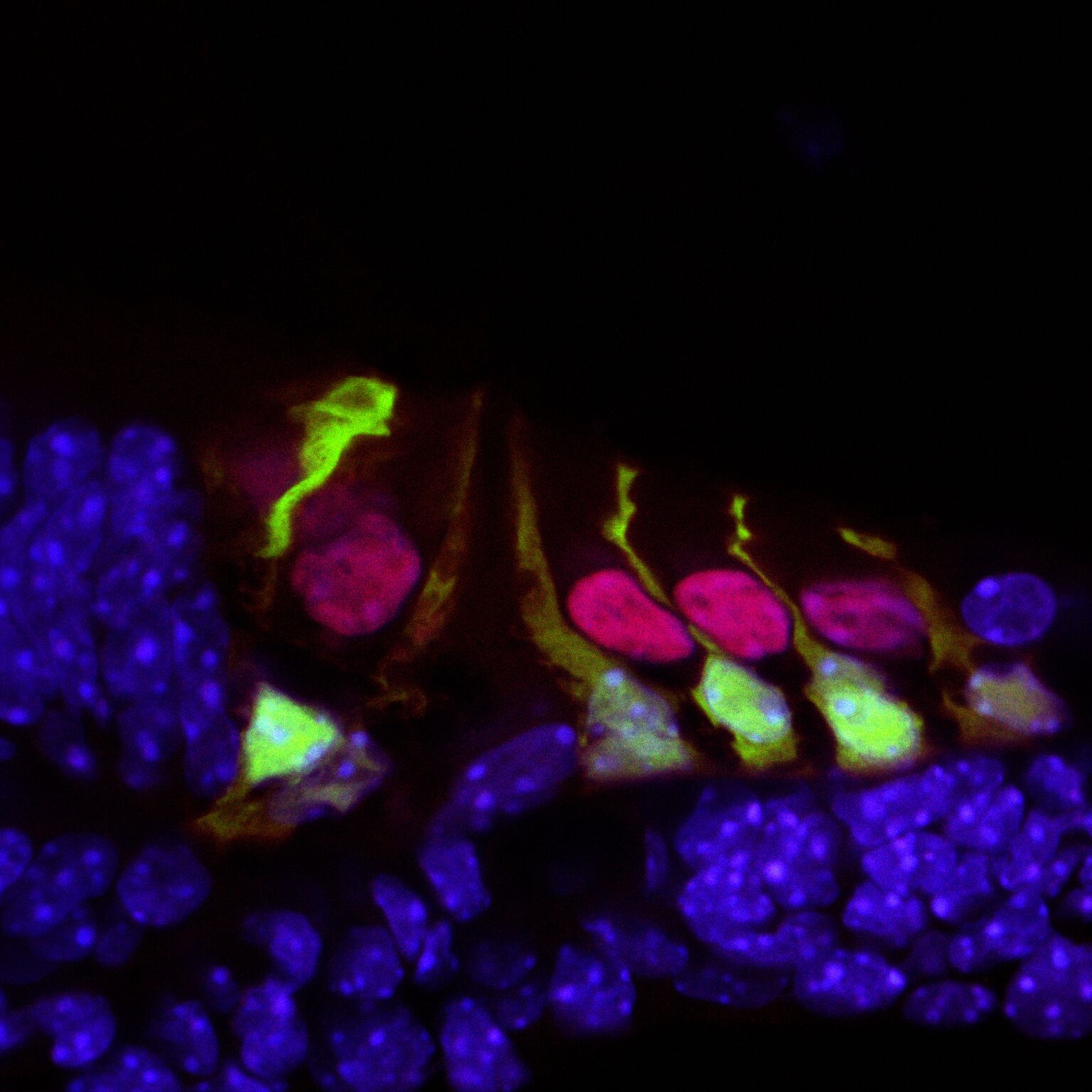
We found C1QL1 expression in the cochlear tissue of adult mice, but not in neonatal or developing mice, indicating that the protein is not involved with the development of any aspect of the auditory system. This developmental regulation is surprising as both C1QL1 and the related C1QL3 have synaptogenic functions.
Study: For better research results, let mice be mice
A new study from the University at Buffalo suggests that the established practice of socially isolating mice for such purposes might actually make them poor research models for humans, and a simple shift to a more realistic social environment could greatly improve the utility of the future studies.
Pinpointing How the Temporal Processing of Nerve Cell Signals Is Important in Hearing
In our study published in the Journal of Neurophysiology, we examined the temporal processing of knockout mice whose cholinergic signaling is disrupted compared with wild-type mouse controls. Findings underscore the importance of cholinergic signaling in types of neurodevelopmental and auditory processing disorders.
Improved TMC1 Gene Therapy Restores Hearing and Balance in Mice
Half of all inner ear disorders, which have a negative impact on hearing and/or balance, are caused by genetic mutations. A study published in January 2019 in Nature Communications demonstrates the effectiveness of a gene therapy targeting one specific gene mutation, TMC1 (transmembrane channel-like 1).
Headlines in Hearing Restoration
By Yishane Lee
The cornerstone of Hearing Health Foundation for six decades has been funding early-career hearing and balance researchers through its Emerging Research Grants (ERG) program. Many ERG scientists have gone on to obtain prestigious National Institutes of Health (NIH) funding to continue their HHF-funded research; since 1958, each dollar awarded to ERG scientists by HHF has been matched by NIH investments of more than $90. Within the scientific community, ERG is a competitive grant awarded to the most promising investigators, and we’re always especially pleased when our ERG alumni who are now also members of or affiliated with our Hearing Restoration Project consortium make headlines in the mainstream news for their scientific breakthroughs.
Hair cells in the mouse cochlea courtesy of the lab of Hearing Restoration Project (HRP) member Andy Groves, Ph.D., Baylor College of Medicine.
Ronna Hertzano, M.D., Ph.D. (2009–10): Hearing Restoration Project consortium member Hertzano, an associate professor at the University of Maryland School of Medicine, and colleagues identified a gene, Ikzf2, that acts as a key regulator for outer hair cells whose loss is a major cause of age-related hearing loss. The Ikzf2 gene encodes helios, a transcription factor (a protein that controls the expression of other genes). The mutation of the gene in mice impairs the activity of helios in the mice, leading to an outer hair cell deficit.
Reporting in the Nov. 21, 2018, issue of Nature, the team tested whether the opposite effect could be created—if an abundance of helios could boost the population of outer hair cells. They introduced a virus engineered to overexpress helios into the inner ear hair cells of newborn mice, and found that some mature inner hair cells became more like outer hair cells by exhibiting electromotility, a property limited to outer hair cells. The finding that helios can drive inner hair cells to adopt critical outer hair cell characteristics holds promise for future treatments of age-related hearing loss.
Patricia White, Ph.D. (2009, 2011), with Hearing Restoration Project member Albert Edge, Ph.D.: White, a research associate professor at the University of Rochester Medical Center, Edge, a professor of otolaryngology at Massachusetts Eye and Ear and Harvard Medical School, and team have been able to regrow the sensory hair cells found in the mouse cochlea. The study, published in the European Journal of Neuroscience on Sep. 30, 2018, builds on White’s prior research that identified a family of receptors called epidermal growth factor (EGF) that is responsible for activating supporting cells in the auditory organs of birds. When triggered, these cells proliferate and foster the generation of new sensory hair cells. In mice, EGF receptors are expressed but do not drive regeneration of hair cells, so it could be that as mammals evolved, the signaling pathway was altered.
The new study aimed to unblock the regeneration of hair cells and also integrate them with nerve cells, so they are functional, by switching the EGF signaling pathway to act as it does in birds. The team focused on a specific receptor called ERBB2, found in supporting cells. They used a number of methods to activate the EGF signaling pathway: a virus targeting ERBB2 receptors; mice genetically altered to overexpress activated ERBB2; and two drugs developed to stimulate stem cell activity in the eye and pancreas that are already known to activate ERBB2 signaling. The researchers found that activating the ERBB2 pathway triggered a cascading series of cellular events: Supporting cells began to proliferate and started the process of activating other neighboring stem cells to lead to “apparent supernumerary hair cell formation,” and these hair cells’ integration with the network of neurons was also supported.
This was prepared using press materials from the University of Maryland and the University of Rochester. For more, see hhf.org/hrp.
Genome Editing Protects Hearing in Mice
Massachusetts Eye and Ear/Harvard Medical School associate professor Zheng-Yi Chen, D.Phil. (1995 ERG), and colleagues delivered a CRISPR/Cas9 genome editing complex directly into the inner ear hair cells of mice, preventing hearing loss in an animal model of genetic progressive deafness. The CRISPR/Cas9 therapy disabled a mutated form of the gene Tmc1, the first time that a gene editing protein has been ferried directly into the relevant cells to halt progression of genetic hearing loss.
A single-letter mutation in the gene Tmc1 and carrying only one of two copies of the mutated gene both lead to progressive hearing loss in mice and humans. With the mutated gene disabled, the inner ear hair cells survive, and mice otherwise genetically destined to become deaf retained a portion of their hearing. In a report published in Nature in December 2017, the team says that at four weeks, untreated mice were unresponsive to sound below an average of 80 decibels, while treated mice responded to sound at approximately 65 decibels. At eight weeks, treated mice also retained their instinctive physical “startle” response to sudden loud sound, while the untreated mice did not respond. The researchers said delivering the Cas9 protein itself locally, instead of DNA elements that the cell can use to build Cas9, improved the DNA specificity and potential safety of the treatment. —Massachusetts Eye and Ear