By Vicky Chan
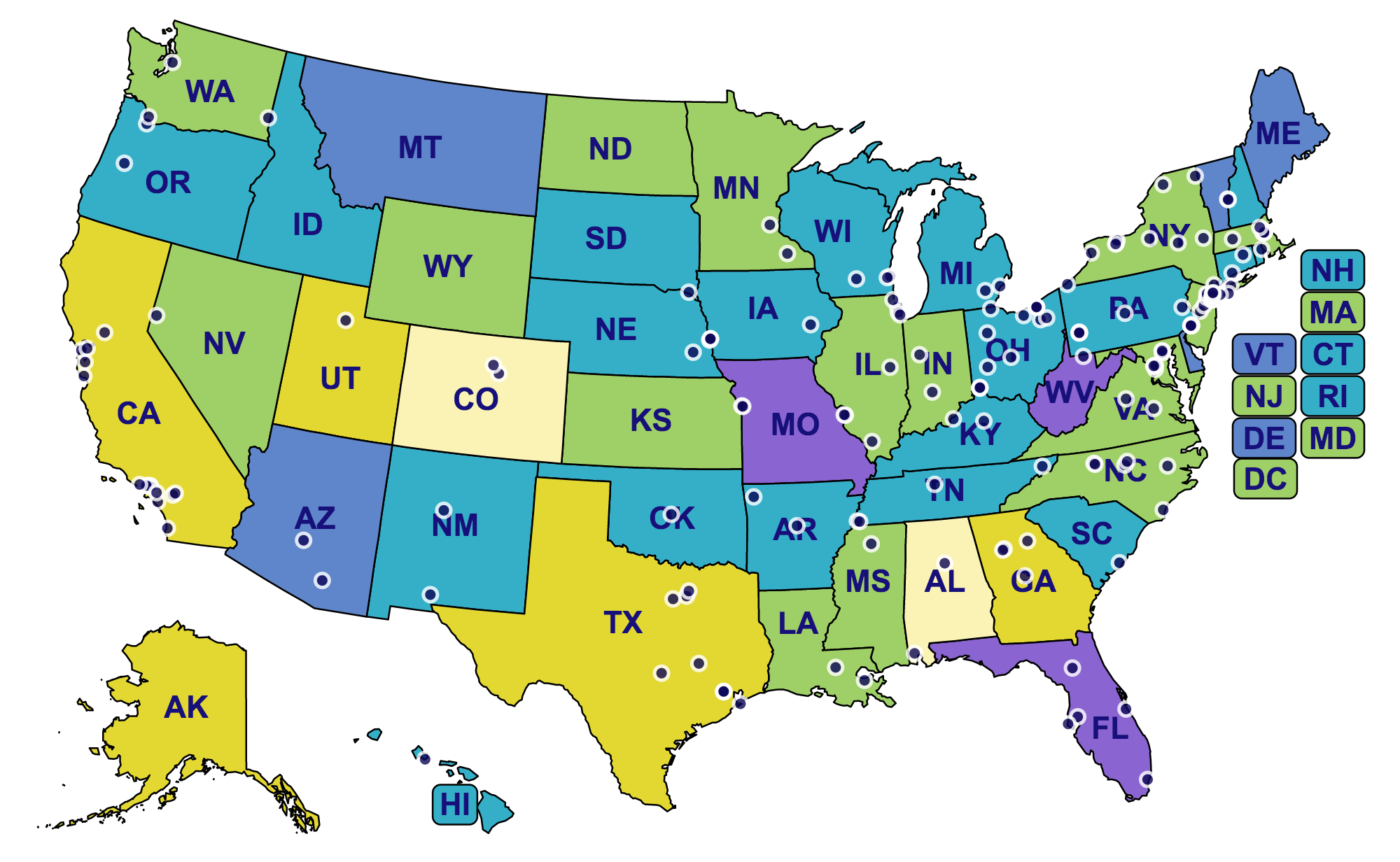
Hearing Health Foundation (HHF) is grateful to the many individuals and organizations who have empowered groundbreaking hearing loss research in the last 60 years. A new interactive map displays every institution in the U.S. where HHF has been fortunate to fund groundbreaking research, yielding outstanding advancements in hearing and balance science. The map also indicates the rates of hearing loss in each state, signaling that additional work is urgently needed.
The colors—light yellow, yellow, green, teal, blue, and purple—represent the rates of hearing loss in each state. The calculations are based off 2015 U.S. Census Data, using estimates from the well-known prevalence of hearing loss among specific demographics. At the lowest end of the range in light yellow, hearing loss affects 13.71% of Colorado’s population. The highest rate was found in Missouri, purple, where the prevalence measured 20.15%. The mean for all states was 18.16%. The numbers signal the significance of hearing loss research.
Nearly all of the institutions on the map represent recipients of the Emerging Research Grants (ERG) who have carried out investigations related to tinnitus, hyperacusis, Ménière's disease, Usher syndrome, hearing loss in children, Central Auditory Processing Disorder, and strial atrophy.
A few institutions are home to the work of the Hearing Restoration Project’s (HRP) domestic consortium members, who focus on investigating hair cell regeneration as a cure for hearing loss and tinnitus. They conduct research at Baylor College of Medicine, Harvard Medical School, Oregon Health & Science University, Stanford University, Stowers Institute, University of Maryland, University of Michigan, University of Southern California, University of Washington, and Washington University.
By mid-year, the institutions corresponding to HHF’s newly formed Ménière's Disease Grants (MDG) program will be added to the map.
HHF envisions a world in which no one lives with hearing loss and tinnitus—until this is realized, we’ll do everything we can to put more innovative hearing loss research on the map.